Chemical Engineering - (CH) 2018 GATE Paper (Practice Test) - GATE MCQ
30 Questions MCQ Test - Chemical Engineering - (CH) 2018 GATE Paper (Practice Test)
“When she fell down the _______, she received many _______ but little help.”
The words that best fill the blanks in the above sentence are
“In spite of being warned repeatedly, he failed to correct his _________ behaviour.”
The word that best fills the blank in the above sentence is
For 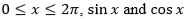 are both decreasing functions in the interval ________.
are both decreasing functions in the interval ________.
The area of an equilateral triangle is √3. What is the perimeter of the triangle?
Arrange the following three-dimensional objects in the descending order of their volumes:
(i) A cuboid with dimensions 10 cm, 8 cm and 6 cm
(ii) A cube of side 8 cm
(iii) A cylinder with base radius 7 cm and height 7 cm
(iv) A sphere of radius 7 cm
An automobile travels from city A to city B and returns to city A by the same route. The speed of the vehicle during the onward and return journeys were constant at 60 km/h and 90 km/h, respectively. What is the average speed in km/h for the entire journey?
A set of 4 parallel lines intersect with another set of 5 parallel lines. How many parallelograms are formed?
To pass a test, a candidate needs to answer at least 2 out of 3 questions correctly. A total of 6,30,000 candidates appeared for the test. Question A was correctly answered by 3,30,000 candidates. Question B was answered correctly by 2,50,000 candidates. Question C was answered correctly by 2,60,000 candidates. Both questions A and B were answered correctly by 1,00,000 candidates. Both questions B and C were answered correctly by 90,000 candidates. Both questions A and C were answered correctly by 80,000 candidates. If the number of students answering all questions correctly is the same as the number answering none, how many candidates failed to clear the test?
If �� 2 + �� − 1 = 0 what is the value of
In a detailed study of annual crow births in India, it was found that there was relatively no growth during the period 2002 to 2004 and a sudden spike from 2004 to 2005. In another unrelated study, it was found that the revenue from cracker sales in India which remained fairly flat from 2002 to 2004, saw a sudden spike in 2005 before declining again in 2006. The solid line in the graph below refers to annual sale of crackers and the dashed line refers to the annual crow births in India. Choose the most appropriate inference from the above data.
Consider the following two equations:
The above set of equations is represented by
The fourth order Runge-Kutta (RK4) method to solve an ordinary differential equation
For a special case when the function f depends solely on x, the above RK4 method reduces to
A watch uses two electronic circuits (ECs). Each EC has a failure probability of 0.1 in one year of operation. Both ECs are required for functioning of the watch. The probability of the watch functioning for one year without failure is
The figure which represents (x in radians) is
The terminal velocity of a spherical particle in gravitational settling under Stokes’ regime varies
Critical speed of a ball mill depends on
Economy of evaporators used for concentrating sugarcane juice is
Segmental baffles in a 2-4 shell and tube heat exchanger
In connection with petroleum refining, identify the incorrect statement among the following options.
Polyvinyl chloride is produced by
The molecular formula of the predominant chemical compound in commercial sugar is
Two packed towers are designed for the same mass velocity of the gas. The first has liquid and gas flow rates of 30 kg/s and 1.2 kg/s, respectively, while the corresponding flow rates in the second tower are 67.5 kg/s and 1.8 kg/s. The ratio of the design diameter of the wider tower to that of the narrower tower is
The Annual Fixed Charges (AFC) and Annual Utilities Costs (AUC) of a distillation column being designed are expressed in terms of the reflux ratio (R) as
The reflux ratio (Ropt) for optimizing the total cost of the distillation column may be found by solving
Consider the following properties:
(P) temperature
(Q) specific gravity
(R) chemical potential
(S) volume
The option which lists ALL the intensive properties is
Liquid phase isomerization of o-xylene to p-xylene using a zeolite catalyst was carried out in a CSTR. Three sets of kinetic data at different temperatures and stirring speeds were obtained as shown below.
The operating condition at which the reaction rate is not controlled by external mass transfer resistance is
Choose the correct statement In viscose rayon manufacturing process,
The reactant (M) is converted into product (N) in the presence of catalyst in a fixed bed reactor. All the flow rates (F, G, H, P and R) in mol/s, and mole fraction of reactant (M) in these streams (xF, xG, xH, xP and xR) are shown in the diagram.
The overall fractional conversion is
A first-order process having a transfer function, is controlled by a proportional controller with gain of 3.2 . The process time constant is in minutes. Addition of the integral control action with an integral time constant of 5 minutes leads to increase in
According to the surface renewal theory, the unit of fractional rate of surface renewal is
For absorption of H2S from a mixture with hydrocarbon vapour into an aqueous alkanolamine solution, the liquid phase mass transfer resistance is














