NEET Exam > NEET Tests > Chemistry: Topic-wise Test- 8 - NEET MCQ
Chemistry: Topic-wise Test- 8 - NEET MCQ
Test Description
30 Questions MCQ Test - Chemistry: Topic-wise Test- 8
Chemistry: Topic-wise Test- 8 for NEET 2025 is part of NEET preparation. The Chemistry: Topic-wise Test- 8 questions and answers have been prepared
according to the NEET exam syllabus.The Chemistry: Topic-wise Test- 8 MCQs are made for NEET 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Chemistry: Topic-wise Test- 8 below.
Solutions of Chemistry: Topic-wise Test- 8 questions in English are available as part of our course for NEET & Chemistry: Topic-wise Test- 8 solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Chemistry: Topic-wise Test- 8 | 31 questions in 31 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Chemistry: Topic-wise Test- 8 - Question 1
The molal depression constant for water is 1.86. The depression constant for 100g 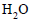 is
is
Chemistry: Topic-wise Test- 8 - Question 2
Urea is added to 2 litre of water to such an extent that 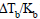 becomes equal to 1/100. The weight of urea added is
becomes equal to 1/100. The weight of urea added is
Chemistry: Topic-wise Test- 8 - Question 3
The moleucular weight of NaCl (degree of dissociation= x) determined by the osmotic pressure method, is found to be different from its actual molecular wieght (M). Which of the following relationship is correct ?
Chemistry: Topic-wise Test- 8 - Question 4
Phenol associates in benzene to produce double moleucles. To what degree phenol assicates if van’t Hoff factor is 0.54 ?
Detailed Solution for Chemistry: Topic-wise Test- 8 - Question 4
Chemistry: Topic-wise Test- 8 - Question 5
Dry air is bubbled successively through (i) a solution,(ii) its solvant, and (iii) through 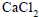 The lowering of vapour pressure of the solvant due to the addition
The lowering of vapour pressure of the solvant due to the addition
of solute is equal to
of solute is equal to
Chemistry: Topic-wise Test- 8 - Question 6
At what temperature does an aqueous solution containing 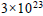 molecules of a noneelectrolyte substance in 250g of water freeze ?
molecules of a noneelectrolyte substance in 250g of water freeze ? 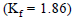
Chemistry: Topic-wise Test- 8 - Question 7
The vapour pressure of a solvent decreased by 10mmHg when a nonvolatile solute was added to the solvent. The mole fraction of the solute in the solution
is 0.2. What would be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mm Hg
is 0.2. What would be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mm Hg
Chemistry: Topic-wise Test- 8 - Question 11
Chemistry: Topic-wise Test- 8 - Question 13
Vapours of an alcohol were passed over red hot copper. It gave an olefin. The alcohol is
Detailed Solution for Chemistry: Topic-wise Test- 8 - Question 15
Chemistry: Topic-wise Test- 8 - Question 16
Increasing order of acid strength among tertiarybutanol, isopropanol and ehanol is
Detailed Solution for Chemistry: Topic-wise Test- 8 - Question 16
Chemistry: Topic-wise Test- 8 - Question 17
How many isomeric alcohols are possible with formula 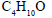
Chemistry: Topic-wise Test- 8 - Question 18
Iodoform gives a precipitate with on heating with but chloroform does not because
Chemistry: Topic-wise Test- 8 - Question 22
Methyl bromide and Ethyl bromide is mixed with equal proportions and the mixture is treated with metallic sodium. Which of the following is not expected to form
Chemistry: Topic-wise Test- 8 - Question 23
Aryl halides are less reactive towards neucliophilic substitution reactions as compared to alkyl halides due to
Chemistry: Topic-wise Test- 8 - Question 24
Propene is allowed to react with HI. The product (A) is then treated with to give a new product (B)
Chemistry: Topic-wise Test- 8 - Question 26
Which of the following statements about phenol is correct?
Chemistry: Topic-wise Test- 8 - Question 27
The utility of the polymers in various fields is due to their mechanical properties like tensile strength , elasticity, toughness etc. These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Vulcanized rubber is an example of
Vulcanized rubber is an example of
Chemistry: Topic-wise Test- 8 - Question 28
The utility of the polymers in various fields is due to their mechanical properties like tensile strength , elasticity, toughness etc.These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
The molecular forces of attraction are weakest in
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
The molecular forces of attraction are weakest in
Chemistry: Topic-wise Test- 8 - Question 29
The utility of the polymers in various fields is due to their mechanical properties like tensile strength , elasticity, toughness etc.These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Which of the following have usually a linear structure?
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Which of the following have usually a linear structure?
Chemistry: Topic-wise Test- 8 - Question 30
The utility of the polymers in various fields is due to their mechanical properties like tensile strength , elasticity, toughness etc.These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Nylon-6 which is fibre is made from
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Nylon-6 which is fibre is made from
View more questions
Information about Chemistry: Topic-wise Test- 8 Page
In this test you can find the Exam questions for Chemistry: Topic-wise Test- 8 solved & explained in the simplest way possible.
Besides giving Questions and answers for Chemistry: Topic-wise Test- 8, EduRev gives you an ample number of Online tests for practice
Download as PDF
















