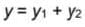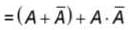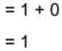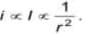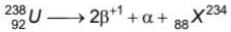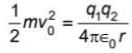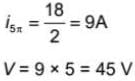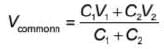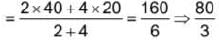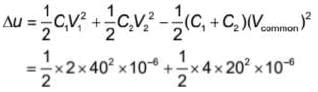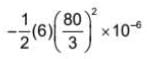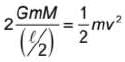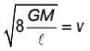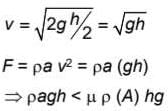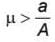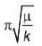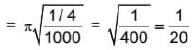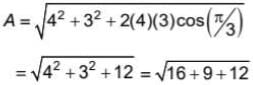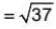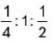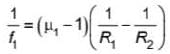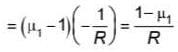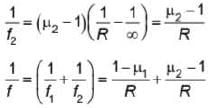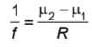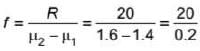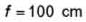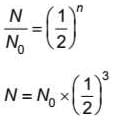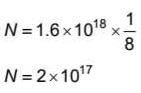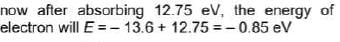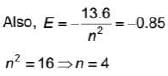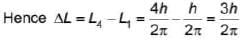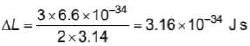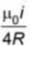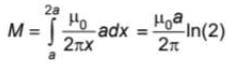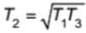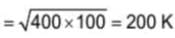NEET Practice Test - 17 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 17
The mass and volume of a body are 6.237 g and 3.5 cm3, respectively. The density of the material of the body in correct significant figures is
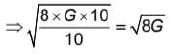
The magnitude of velocity of a body is given by V =  m/s, then average speed of body between 2s to 6s
m/s, then average speed of body between 2s to 6s
 m/s, then average speed of body between 2s to 6s
m/s, then average speed of body between 2s to 6sIf R and h represents the horizontal range and maximum height respectively of an oblique projectile, then 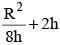 represents
represents
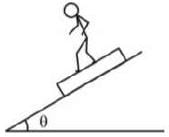
Rohan is running down with some acceleration on a plank kept on fixed inclined plane as shown in figure. Which of the following cases are possible?
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut-off voltage and saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6m away from the photoelectric cell, then which of the following are true?
After the emission of one α -particle followed by two +1β particles from 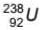 , the number of neutrons in the newly formed nucleus is
, the number of neutrons in the newly formed nucleus is
An α -particle of energy 5 MeV is scattered through 180o by a fixed uranium nucleus. The closet distance is in order of
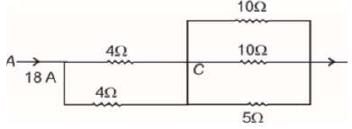
Five resistors of resistances as indicated in the figure are connected together. If a current of 18 A enters into the resistance network at A, then the potential difference across 5Ω resistor will be
A capacitor of capacitance 2 μF is charged to 40 V and another capacitor of capacitance 4 μF is charged to 20 V. If the capacitors are connected together in same polarity, then the energy lost in reorganisation of charge will be
In space two point masses of mass 10 kg each are fixed. The masses are separated by a distance 10 m. Another point mass of mass 1 kg is to be projected from the point at midpoint of line joining the fixed masses such that it escapes to infinity. The minimum speed of the projection is
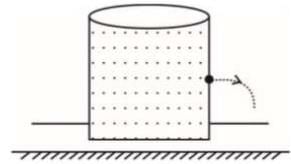
A container of height h is completely filled with water. The container is placed on a frictional surface with coefficient of friction μ and a small hole is punctured at a depth h/2 on container wall. If area of hole is a and area of base of container is A (a << A), then the value of μ for which the container remains stationary is (g = 10 m/s2)
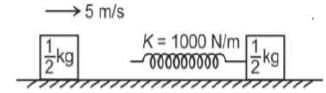
A 1/2 kg block moving with 5 m/s strikes a spring of force constant 1000 N/m attached to another 1/2 kg block at rest. Kept on a smooth floor. The time for which the rear moving block remain in contact with the spring will be (Assume π = √10 )
If two disturbances represented by equation y1 = 4sin(ωt) and y2 = 3sin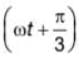 interfere at a point. Then the amplitude of the resulting disturbance will be.
interfere at a point. Then the amplitude of the resulting disturbance will be.
A charged Q is divided into two charge q1 and q2, separated by a distance r. The force of repulsion between them will be maximum when
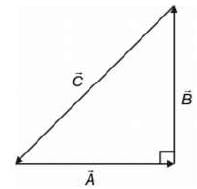
If 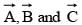 are three vectors of magnitudes 12 units, 5 unit and 13 unit. And
are three vectors of magnitudes 12 units, 5 unit and 13 unit. And 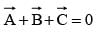 then angle between
then angle between is
is
A sonometer wire of length 280 cm is divided into 3 segments having fundamental frequencies in the ratio 4: 1 : 2. The lengths of the segments are
Length, breadth and height of a cuboid are measured as 1.61 m, 2.2 m, and 3.1 m. The volume of cuboid up to correct number of significant figures is
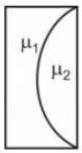
A plano concave lens fits into a plano convex lens. Their plane surfaces are parallel to each other as shown in the figure. If μ1 = 1.4, μ2 = 1.6 and radius of curvature R=20 cm, then the focal length of the combination is
A radioactive sample has 1.6 x1018 radioactive nuclei at a certain instant. After three half lives the number of nuclei that will remain undecayed is
Hydrogen atom in ground state absorbs 12.75 eV of energy. The orbital angular momentum of the electron is increased by
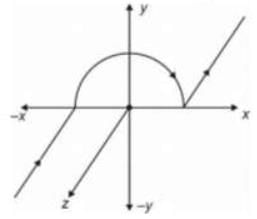
A wire carrying current i is shaped as shown in the figure. The magnetic field at the origin is (R: Radius of circular section)
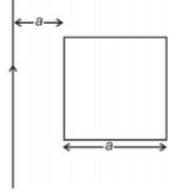
A square loop and a long straight wire are situated in a common plane such that one edge of the square is parallel to the wire. The mutual inductance between the wire and loop is
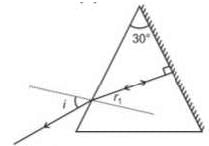
A face of a prism of refracting angle 30o is silvered. A ray of light is incident on the other face at angle of incidence 45o. After refection from the silvered face, ray retraces its path. The index of refraction of prism will be
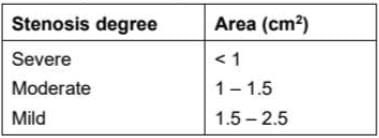
The degree of aortic value stenosis (abnormal narrowing of blood vessels) can be determined by calculating the area A2 of valvular opening and comparing with data given
Consider following aortic flow and blood as incompressible fluid
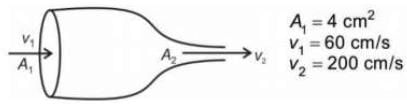
The degree of stenosis in cross-section A2 is
Two carnot engines are operated in succession. The first one, receives heat from a source at T1 = 400 K and rejects to sink at T2 K. The second engine receives heat rejected by first engine and rejects to another sink at T3=100 K. If the efficiency of both the engines are equal then T2 is


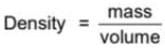
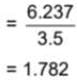
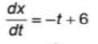
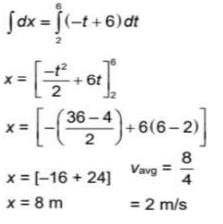

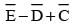 equals
equals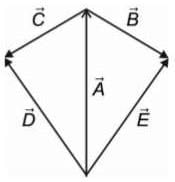



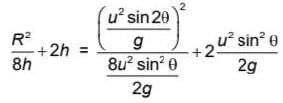
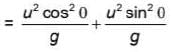
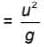

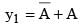 and
and 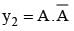 then value of y1+y2 in Boolean algebra is
then value of y1+y2 in Boolean algebra is