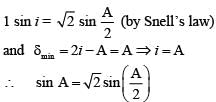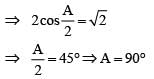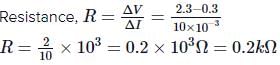NEET Mock Test - 8 - NEET MCQ
30 Questions MCQ Test - NEET Mock Test - 8
A convex lens made up of glass (μ = 1.5) has 20 cm focal length in air (μ = 1). Then its focal length in water (μ = 1.33) will be :
The critical angle of light going from denser medium to rarer medium is θ. If the speed of light in denser medium is v, then speed of light in rarer medium is
For a glass prism of refractive index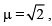 the angle of minimum deviation is equal to the angle of prism, then angle of prism is
the angle of minimum deviation is equal to the angle of prism, then angle of prism is
There is a small object at centre C of a solid glass sphere of refractive index μ. When seen from outside, the dot will appear to be located
A lens behaves as a converging lens in air and a diverging lens in water. The refractive index of the material is
A quartz slab is placed inside glycerine as shown in the figure. The shift of object observed by observer on left side is :
The largest distance of the image of a real object from a convex mirror of focal length 20 cm can be
A hypermetropic person can see the objects beyond 1 m from his eyes. What should be the power of spectacles we should use so that his least distance of clear vision becomes 25 cm
A simple microscope has a focal length of 2.5 cm. Maximum angular magnification of it will be (Given that least distance of clear vision = D = 25 cm)
A normal human eye can see from 25 cm to infinity. If the distance between eye lens and retina is 2 cm, then which of the following is correct?
Stopping potential in photoelectric experiment for a given metal surface
When a transistor is used in common emitter mode as an amplifier
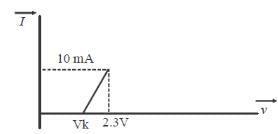
Q. The resistance of a germanium junction diode whose V−I is shown in the figure is (Vk=0.3V)
Input applied to the following circuit will produce which of the following output signal, assuming R1 = R2 and diode is ideal.
If the ratio of amplitudes of two coherent sources producing an interference pattern is 3 : 4, then ratio of intensities at maxima and minima is
The ionization potential of hydrogen atom is − 13.6 V. Photons of energy 12.75 eV are made to incident on sample of H atoms at ground state. How many spectral lines are expected in emitted radiation?
Electromagnetic radiations of intensity I is incident normally on a perfectly reflecting surface. If speed of light is C, then radiation pressure exerted on the surface is
A beam of unpolarised light travelling in air is reflected from a solid surface at an angle of reflection 60°. If the reflected light is plane polarized, then refractive index of the solid is
de-Brogile wavelength associated with a proton accelerated from rest by 10 kV is λ. The de-Brogile wavelength associated with a 10 keV neutron is
Momentum of an alpha particle emitted from a stationary nucleus is. Momentum of the daughter nucleus should be
In a Young’s double-slit experiment, the intensity at a bright fringe is I0. If one of the slits is now covered, the intensity at any point on the screen will be
In a Young’s double-slit experiment, the central bright fringe can be identified
In a Young’s double-slit experiment, if the slits are of unequal width,
In a Young’s double-slit experiment, the fringe width is β. If the entire arrangement is now placed inside a liquid of refractive index μ, the fringe width will become
In a Young’s double-slit experiment, let S1 and S2 be the two slits and C be the centre of the screen. If ∠S1CS2 = θ and λ is the wavelength, the fringe width will be
The penetrating powers of α, β and γ radiations, in decreasing order, are