Physics: Topic-wise Test- 6 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series - Updated 2026 Pattern - Physics: Topic-wise Test- 6
If radiation of allow wavelengths from ultraviolet to infrared is passed through hydrogen agas at room temperature, absorption lines will be observed in the :
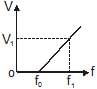
In a photoelectric experiment, the potential difference V that must be maintained between the illuminated surface and the collector so as just to prevent any electron from reaching the collector is determined for different frequencies f of the incident illumination. The graph obtained is shown. The maximum kinetic energy of the electrons emitted at frequency f1 is
In photoelectric effect, stopping potential depends on
In the experiment on photoelectric effect using light having frequency greater than the threshold frequency, the photocurrent will certainly increase when
An electron in hydrogen atom first jumps from second excited state to first excited state and then, from first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons in the two cases by x, y and z, then select the wrong answers :
A particular hydrogen like atom has its ground state binding "energy 122.4 eV. Its is in ground state. Then :
The electron in a hydrogen atom makes a transition n1 ¾→ n2, where n1 & n2 are the principal quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. The possible values of n1 & n2 are :
In hydrogen atom the kinetic energy of electron in an orbit of radius r is given by
According to Bohr model of hydrogen atom, the radius of stationary orbit characterized by the principal quantum number n is proportional to
Select an incorrect alternative:
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
In Bohr model of hydrogen atom, radiation is emitted when the electron
Which of the following is/are deduced from the Rutherford’s scattering experiment?
(1) There are neutrons inside the nucleus.
(2) The sign of the charge of the nuclei is the same as the sign of alpha particles.
(3) Electrons are embedded in the nucleus.
The alpha particle scattering experiment was carried out by:
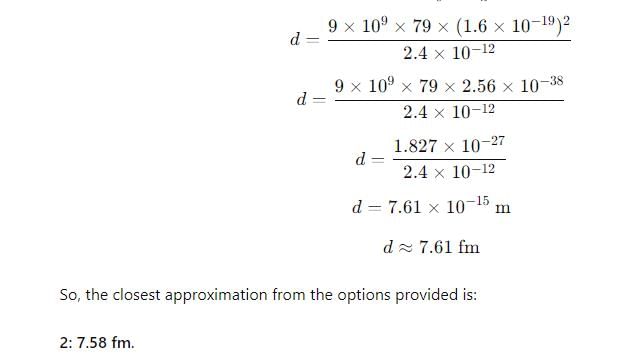
The distance of closest approach when a 15.0 MeV proton approaches gold nucleus (Z = 79) is
The targets used in the alpha particle atomic experiments in the early 1900’s was:
Rutherford’s experiments on scattering of alpha particles proved that:
What percentage of the mass of an atom is concentrated in the nucleus?
Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is
In a nuclear reaction which of the following is conserved
B210has a half life of 5 days. The time taken for seven-eighth of a sample to decay is
If 10% of a substance decays in 10 days, then approximate percentage of substance left after 24 days is
The name of a diode that can be used to provide a variable capacitance is:
Which of the statements is true for p-type semiconductors?
In an unbiased p-n junction, holes diffuse from the p-region to n-region because
|
1 videos|19 docs|71 tests
|


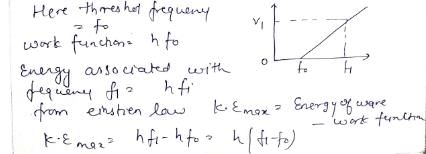

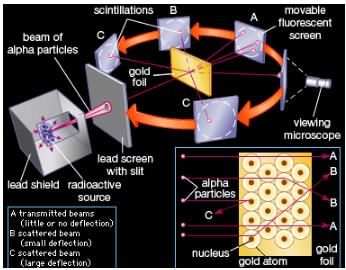 Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. When he shot a beam of alpha particles at a sheet of gold foil, a few of the particles were deflected. He concluded that a tiny, dense nucleus was causing the deflections.
Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. When he shot a beam of alpha particles at a sheet of gold foil, a few of the particles were deflected. He concluded that a tiny, dense nucleus was causing the deflections.















