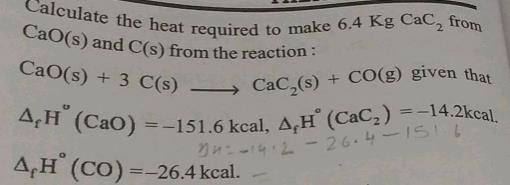NEET Exam > NEET Tests > Test: Chemistry Mock Test - 10 - NEET MCQ
Test: Chemistry Mock Test - 10 - NEET MCQ
Test Description
30 Questions MCQ Test - Test: Chemistry Mock Test - 10
Test: Chemistry Mock Test - 10 for NEET 2025 is part of NEET preparation. The Test: Chemistry Mock Test - 10 questions and answers have been prepared
according to the NEET exam syllabus.The Test: Chemistry Mock Test - 10 MCQs are made for NEET 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Chemistry Mock Test - 10 below.
Solutions of Test: Chemistry Mock Test - 10 questions in English are available as part of our course for NEET & Test: Chemistry Mock Test - 10 solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Chemistry Mock Test - 10 | 45 questions in 45 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Test: Chemistry Mock Test - 10 - Question 2
A salt is heated first with dil. H₂SO₄. No reaction takes place. It may be
Test: Chemistry Mock Test - 10 - Question 3
Which of the following sets of quantum numbers belongs to highest energy?
Test: Chemistry Mock Test - 10 - Question 4
Methyl benzene can be prepared by reacting benzene with bromo-methane in the presence of
Test: Chemistry Mock Test - 10 - Question 7
BCl₃ molecule is planar while NCl₃ is pyramidal because
Test: Chemistry Mock Test - 10 - Question 8
Internal energy change when system goes from state A to B is 40 KJ/mol. If the system goes from A to B by reversible path and returns to state A by irreversible path, what would be net change in internal energy ?
Test: Chemistry Mock Test - 10 - Question 9
Which one of the following informations can be obtained on the basis of Le Chatelier principle ?
Test: Chemistry Mock Test - 10 - Question 10
In the titration between oxalic acid and acidified potassium permanganate, the manganous salt formed catalyses the reaction. The manganous salt is
Test: Chemistry Mock Test - 10 - Question 11
One of the oxidants used with liquid propellants is
Detailed Solution for Test: Chemistry Mock Test - 10 - Question 12
Test: Chemistry Mock Test - 10 - Question 14
Which one of the following represents the correct order of electronegativity
Detailed Solution for Test: Chemistry Mock Test - 10 - Question 14
Test: Chemistry Mock Test - 10 - Question 15
Which of the following is not considered as an organometallic compound?
Test: Chemistry Mock Test - 10 - Question 16
Potassium permanganate oxidises sulphur dioxide to
Test: Chemistry Mock Test - 10 - Question 18
Three faradays of electricity was passed through an aqueous solution of Iron (II) bromide. The weight of iron metal (at. wt = 50) deposited at the cathode is
Test: Chemistry Mock Test - 10 - Question 19
If 0.44 g of a colourless oxide of nitrogen occupies 224 ml at 1520 mm hg and 273oC, then the compound is
Test: Chemistry Mock Test - 10 - Question 21
Which one of the following elements is a non-metal
Test: Chemistry Mock Test - 10 - Question 22
The ion that can be preciitated by HCl as well as H₂S is
Test: Chemistry Mock Test - 10 - Question 23
Which one of the following oxides is most acidic ?
Test: Chemistry Mock Test - 10 - Question 25
Which of the following can give iodometric titration?
Test: Chemistry Mock Test - 10 - Question 27
Which of the following isomers will have the highest boiling point ?
Test: Chemistry Mock Test - 10 - Question 28
The property of hydrogen which distinguishes it from alkali metals is
View more questions
Information about Test: Chemistry Mock Test - 10 Page
In this test you can find the Exam questions for Test: Chemistry Mock Test - 10 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Chemistry Mock Test - 10, EduRev gives you an ample number of Online tests for practice
Download as PDF