Test: Conformational Analysis - 1 - NEET MCQ
10 Questions MCQ Test Chemistry Class 11 - Test: Conformational Analysis - 1
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
The molecular formula C5H12 contains how many isomeric alkanes?
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Which of the following cycloalkanes exhibits the greatest molar heat of combustion per —CH2 — group?
Which of the following correctly ranks the cycloalkanes in order of increasing ring strain per methylene group?
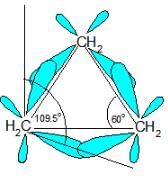
Which,of the following correctly lists the conformations of cyclohexane in order of increasing potential energies?
Which is not true regarding conformers of ethane?
The correct statement concerning conformers of 1,2-dichloroethane is
Which of the following molecule exhibits conformational isomerism ?
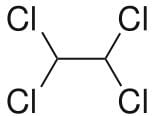
Which is true about conformers of 1,1,2,2-tetrachloroethane?
Which is true about conformers of 1,1,2,2-tetrachloroethane?
a) The most stable conformer has dihedral angle of 60° between all adjacent chlorine atoms
b) In the least stable conformer, two Cl-atoms are eclipsing one another while other two Cl-atoms are eclipsed to hydrogen atoms
c) In the most stable conformer, dihedral angle between hydrogen atoms is 60°
d) The most stable conformer is non-polar
What is relationship between the following Fischer Projections?
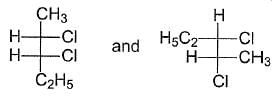
|
108 videos|241 docs|74 tests
|