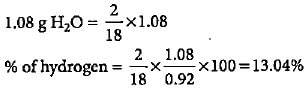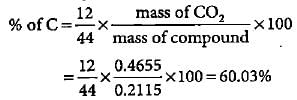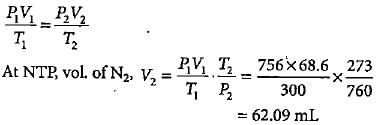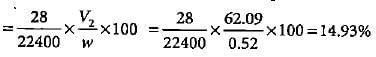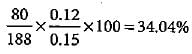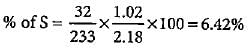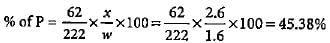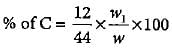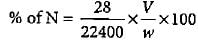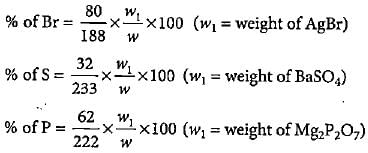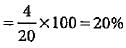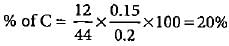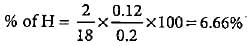Test: Quantitative Analysis (NCERT) - NEET MCQ
10 Questions MCQ Test - Test: Quantitative Analysis (NCERT)
0.92 g of an organic compound was analysed by combustion method. The mass of the U-tube increased by 1.08 g. What is the percentage of hydrogen in the compound?
An organic compound gave 0.4655 g of CO2 on complete combustion. If the mass of the compound taken was 0.2115 g, what is the percentage of C in it?
In Duma'smethod 0.52 g of an organic compound on combustion gave 68.6 mL N2 at 27oC and 76 mm pressure. What is the percentage of nitrogen in the compound?
In kjeldahl's method of estimation of nitrogen, nitrogen is quantitatively converted to ammonium sulphate. It is the treated with the standard solution of alkali. The nitrogen which is present is estimated as:
In Carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 g of AgBr. What is the percentage of bromine in the compound?
2.18 g of an organic compound containing sulphur produces 1.02g of BaSO4. Thepercentage of sulphur in the compound is
1.6 g of an organic compound gave 2.6 g of magnesium pyrophosphate. The percentage of phosphorus in the compound is
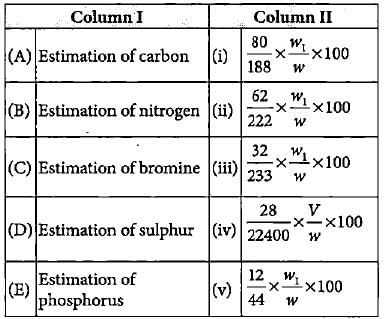
Match the column I with column II in which formula for estimation of an element is given and mark the appropriate choice.
0.2 g of an organic compound contains C, H and O.On combustion, it yields 0.15 g CO2 and 0.12 g H2O.Ihe percentage of C, H and O respectively is