Test: Name Reaction Level - 1 - Chemistry MCQ
30 Questions MCQ Test Organic Chemistry - Test: Name Reaction Level - 1
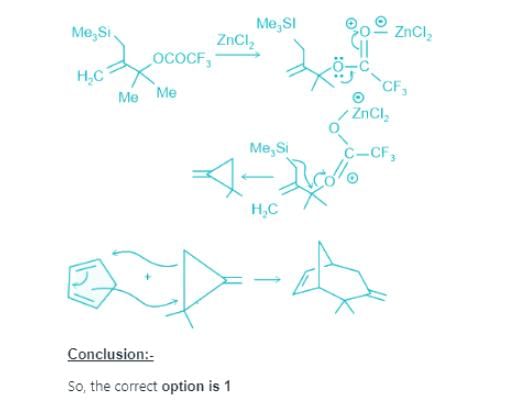
Predict the product of the following reaction sequence:
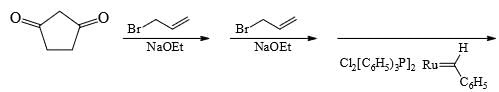

Predict the product of the following reaction sequence.
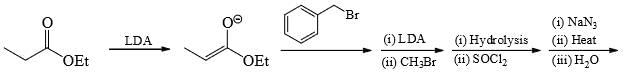

The major product formed in the following reaction is:
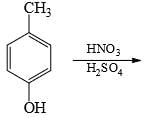

Choose the reaction sequence that could be used to perform the following transformation:
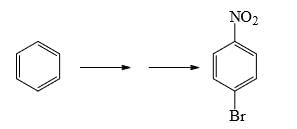
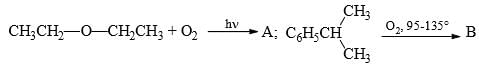
What could be reagents A and B for the following reactions:
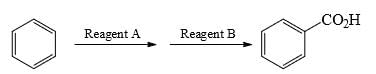
What could be the reagent to complete the following reaction:
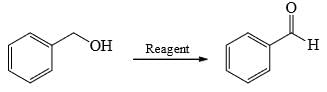
Choose order that has the following compounds correctly arranged with respect to increasing rate of reaction with LiAlH4 (most reactive compound on the right).
Choose the reaction(s) that will not proceed as shown hereunder:
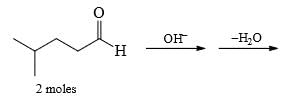
Predict the product of the following aldol condensation:
Which of the following is most reactive towards aqueous HBr:
Ethylbenzene when treated with chlorine in presence of light mainly gives:
The following alcohol is treated with Conc. H2SO4, the major product obtained is:

The major product formed in the following reaction is:

Nitrobenzene can be reduced to aniline by:
(I) H2/Ni
(II) Sn/HCl
(III) Zn/NaOH
(IV) LiAlH4
1-Methylcyclopentene can be converted into 2-methylcyclopentanol by:
2-Methylpropanol-2 can be obtained by the acid-catalyzed hydration of:
The major product formed in the following reaction is:
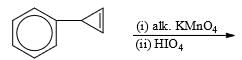
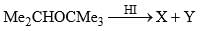
Predict the nature of product and the type of reaction involved in their formation.
Anisole is treated with HI under two different conditions:
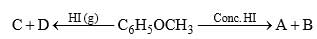
The nature of A and D will be
Predict the compounds A and B in the following reactions:
Which of the following statement is true regarding amount of AlCl3 required during Friedel-Craft acetylation using acetyl chloride or acetic anhydride:
Which of the following gives effervescences of CO2 with NaHCO3 solution:
2,4,6-Trinitrophenol can be prepared in good yield:
|
44 videos|102 docs|52 tests
|



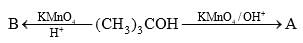
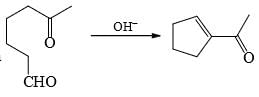 is an example of:
is an example of: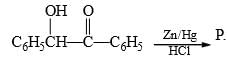 Here, P should be:
Here, P should be: