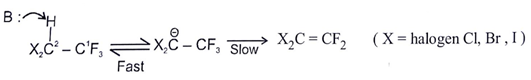Test: Elimination Reaction - Chemistry MCQ
15 Questions MCQ Test Organic Chemistry - Test: Elimination Reaction
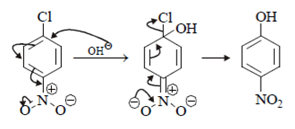
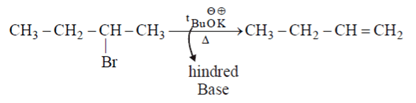
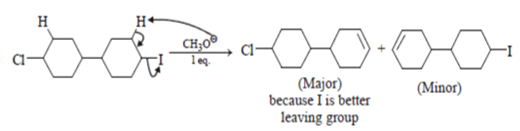
Which of the following is incorrect statement for the giver reaction?
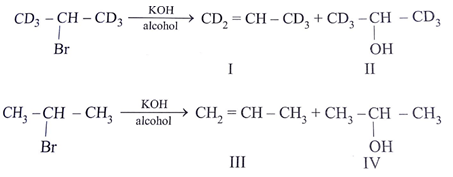

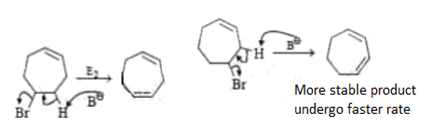
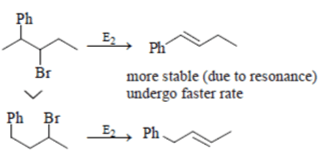
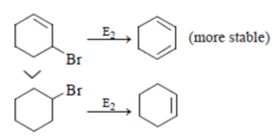
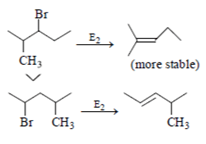
Which of the following order is incorrect for the rate of E2 reaction?
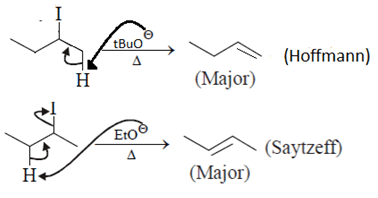
Which of the following statement (s) is/are true about the following eliminations?

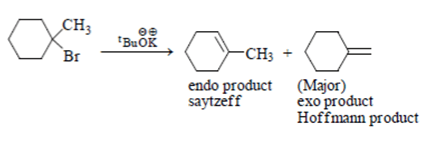
(1) Hoffmann product is major product in I (2) Saytzeff product is major product in I (3) Hoffmann product is major product in II (4) Saytzeffproduct is major product in II

(1) Hoffmann product is major product in I (2) Saytzeff product is major product in I (3) Hoffmann product is major product in II (4) Saytzeffproduct is major product in II
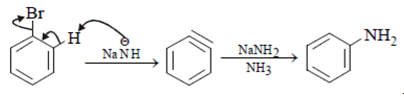
Which of the following reaction will not show addition elimination mechanism?
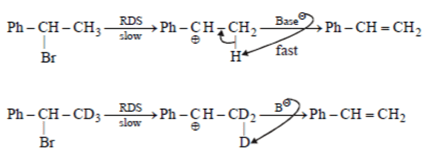
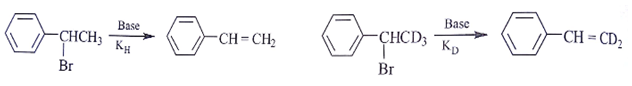
Assuming both the reactions as E1, where will the expected ratio between KH/KD lies between?
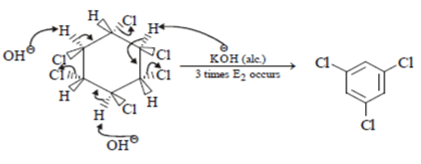
When the all-cis isomer of C6H6Cl6 (2, 3, 4, 5, 6-Hexachlorocyclohexane) is heated with alc.KOH, what will be the most probable product?
In which reaction product formation takes place by hoffmann rule?
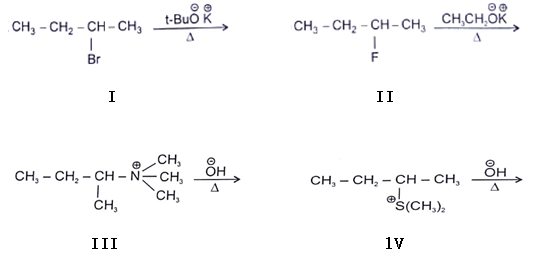
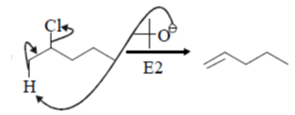
Predict the product for the following elimination reaction.
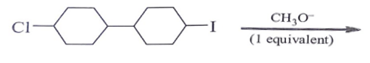
Which of the following statements is correct for alkyl halide?
Which of the following statement is correct regarding the following reaction?
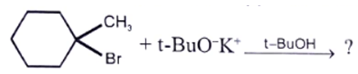
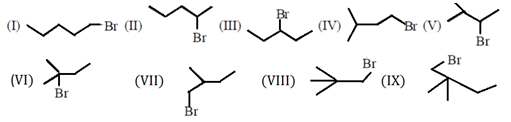
Which incorrect about alkyl bromide having molecular formula C5H11Br?
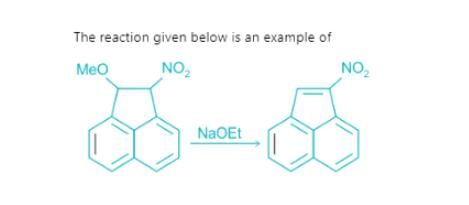
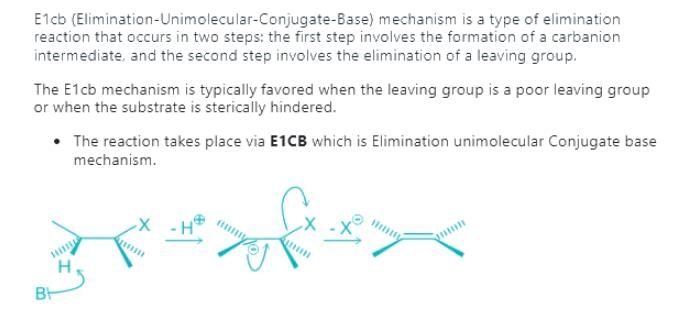
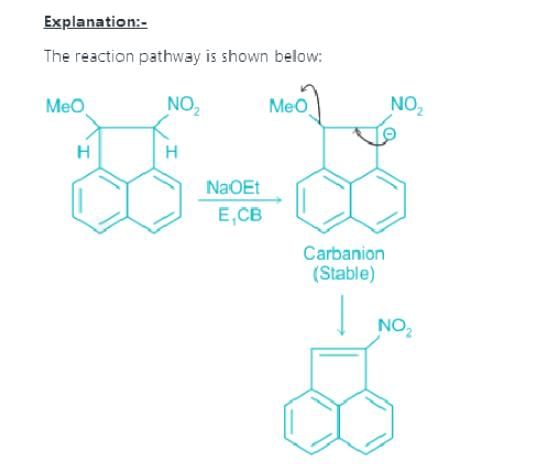
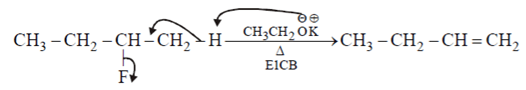
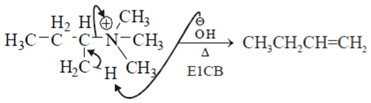
Which of the following is not the examples of E1CB reaction?
|
44 videos|102 docs|52 tests
|