UP PGT Chemistry Mock Test - 1 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 1
Electronic configuration of the element having atomic number 24.
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The incorrect statement concerning E1 reaction is
A current of 0.1A was passed for 2hr through a solution cuprocyanide and 0.3745g f copper was deposited on the cathode. Calculate the current efficiency for the copper deposition.
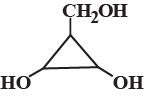
The number of optically active optical isomers of the compound is:
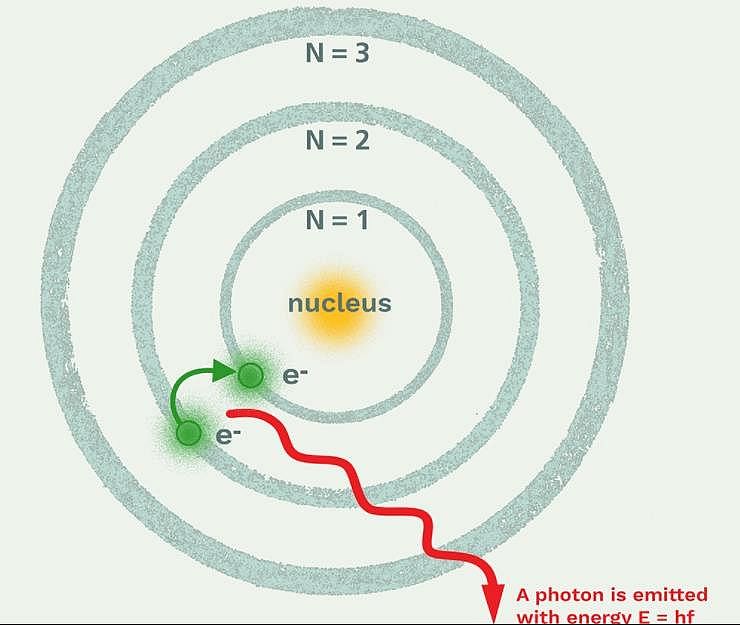
Due to the presence of electrons in the inner shells, the electron in the outer shell will not experience the full positive charge of the nucleus (Ze). This is known as
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of vapour at one atmosphere is
[IIT JEE 2004]
Which property of colloids is applied in rubber plating & sewage disposal?
Arrange the following compounds in decreasing order of their acid strength: i) trichloroacetic acid ii) trifluoroacetic acid iii) acetic acid and iv) formic acid
In the gas phase water is a bent molecule with a bond angle of
In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Which of the following concentration factors is affected by change in temperature?
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?
Which one of the following statement is false?
[AIEEE-2004]
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The most appropriate reagents that can bring about the following transformation is
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The equilibrium which is not affected by volume change at constant temperature is
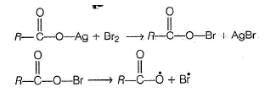
The yield of alkyl bromide obtained as a result of heating the dry silver salt of carboxylic acid with bromine in CCI4 is
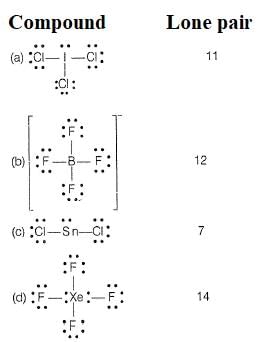
Which of the following molecule/species has the minimum number of lone pairs?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the following property decreases down the group in the halogens?
How many moles of magnesium phosphate, Mg3 (PO4)2 will contain 0.25 mole of oxygen atoms?
On mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane. This alkane could be -
[AIEEE-2003]
In the structure of NaCI given below, ratio rNa+/rcl- is
|
30 tests
|



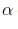 - position.
- position.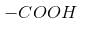
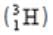 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons.