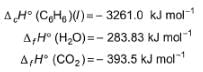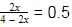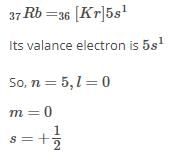UP PGT Chemistry Mock Test - 6 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 6
Acetic acid has Ka = 1.8 X 10 – 5 while formic acid had Ka = 2.1 X 10–4. What would be the magnitude of the emf of the cell
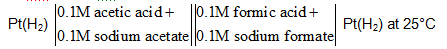
Noble gases are inert and do not form compounds like other elements because of their:
Which of the following elements are called representative elements?
Elements of which group are known as ore forming elements?
Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, l = 1 and 2 are, respectively [AIEEE- 2004]
Energy of the electron in nth orbit is given by E Wavelength of light required to excite an electron in an H-atom from level n = 1 to n = 2 will be (h = 6.62 x 10-34 J s ; c = 3.0 x 108ms -1)
[AIEEE 2012]
Oxygen exhibits +2 oxidation state in the compound:
Calculate resonance energy of N2O from the following data
ΔfH° (N2O) = 82 kJ mol-1
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction,
Presence of particulate matter in polluted air catalyses the oxidation of sulphur dioxide to:
As you move from left to right across the periodic table:
How many valence electrons does a carbon atom have?
Comprehension Type
Direction (Q. Nos. 23-27) This section contains a paragraph, describing theory, experiments, data, etc.
Five questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
A chemist isolated a compound A with molecular formula C7H13Br. A undergoes very fast SN1 reaction. Spectroscopic evidence indicated that compound A has the following structural characteristics : It contains five sp3-hybridised carbon atoms. Among those five sp3 carbon atoms, three are methyl groups, one CH2 group and one CH group.
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
Q.
How many stereoisomers are possible for A?
The greenhouse effect is thought to be the cause of:
In a fuel cell, following reactions takes place and electricity is produced.
Anodic
H2+2OH- → 2H2O + 2e-
Cathodic
O2+2H2O + 4e- → 4OH-
If 100.8 L of H2 at STP reacts in 96500 s,what is the average current produced (in ampere)?
Study the following reactions.
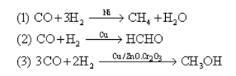
Which characteristic of catalyst is represented by them.?
The more positive the value of E0, the greater is the tendency of the species to get reduced. Using the standard electrode potential of redox couples given below find out which of the following is the strongest oxidising agent.
E0values : Fe3 + / Fe2+ = +0.77; I2(s)/l- = +0.54; cu2+/ Cu = +0.34; Ag+ / Ag = +0.80V
What is the atomic number of the element unniloctium?
For an aqueous solution, freezing point is _0.186ºC . Boiling point of the same solution is
(Kƒ = 1.86º K mol-1 kg) and Kb = 0.512º K mol-1 kg)
[AIEEE-2002]
Identify the pair of enantiomers amongst the given pairs:
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is
[JEE Main 2013]
Sodium pentacyanonitrosylferrate(II) is also called?
|
30 tests
|