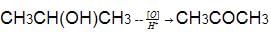UP PGT Chemistry Mock Test - 7 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 7
Predict the major organic product in the following reaction.
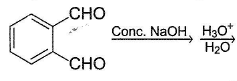
Among the alkenes which one produces tertiary butyl alcohol on acid hydration
How many structural isomers are possible with molecular formula C4H10O ?
Drugs which can block the binding site of the enzyme and prevent the binding of substrate, or can inhibit the catalytic activity of the enzyme are called as:
The pKa of a weak acid (HA) is 4.5. The pOH of an aqueous buffered solution of HA in which 50% of the acid is ionized is:
Given, BE of = 498.8 kJ mol-1
BE of (O—O) in ozone = 302.3 kJ mol-1
Q. What is enthalpy change of the reaction
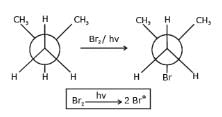
What is the major bromination product in the following reaction?
Different kinds of bonds and interaction present within CuSO4 • 5H2O. They can be
I. σ-bond
II. π-bond
III. coordinate bond
IV. electrostatic force of attraction
V. H-bond due to dipole-dipole interaction
VI. H-bond due to ion-dipole interaction
Select the correct types of bonds/interactions.
Q. Which of the following species contain at least one atom that violates the octet rule?
The salt which finds uses in qualitative inorganic analysis is -?
Identify the element belonging to third period and 17th group of the periodic table.
One of the following rubbers is used in making oil seals, tank lining, etc.
A hydrogen like species in fourth orbit has radius 1.5 times that of Bohr's orbit. In neutral state, its valence electron is in
A __________ overlap doesn’t result in the formation of a bond.
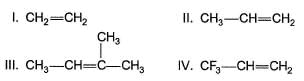
An alkene on treatment with meta chloro perbenzoic acid undergoes epoxidation reaction to give oxirane. What is the correct order of increasing reactivity of the following in the above mentioned epoxidation reaction?
In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3 . Taking kƒ for water as 1.85, the freezing point of the solution will be nearest to _
[AIEEE-2003]
The treatment of CH3MgX with produces
[AIEEE 2008]
The number of lone pairs of electrons on SF4, CF4 and XeF4 are:
Adiponitrile is manufactured electrolytically from acrylonitrile
CH2 = CHCN → CN – (CH2)4 – CN
How many kg of adiponitrile (molecular mass = 108) is produced in 9.65 hr using a current of 3750 A with 80% efficiency
|
30 tests
|



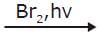 Major Product
Major Product