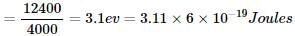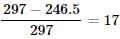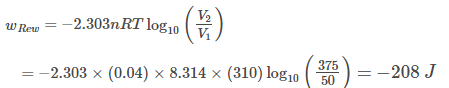UP PGT Chemistry Mock Test - 10 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 10
Which of the following liquid pairs shows a positive deviation from Raoult's law ?
[AIEEE-2004]
Electron gain enthalpy values of noble gases are positive because:
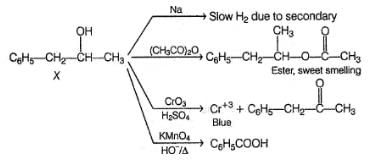
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
If X is treated with HCI in the presence of ZnCI2, the major product would be
A complex of platinum, ammonia and chloride produces four ions per molecule in the solution. The structure consistent with the observation is :
With respect to electron affinity, which statement applies to the halogens?
Value of for SrCl2 in water at 25°C from the following data:

Fermentation of Glucose to alcohol undergoes in presence of :
What is the process of separating emulsion into its constituents called?
An element with atomic number will form a basic oxide________
What is scattering of light by mist on head light of vehicles called?
A photon of 4000 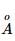 is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is:
is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is:
A piston filled with 0.04 mole of an ideal gas expands reversibly from 50.0 mL to 375.0 mL at constant temperature of 37.0°C. As it does, it absorbs 208 J of heat. The value of q and W for the process will be (R = 8.314 J mol-1 K-1, In 7.5 =2.01)
[JEE Main 2013]
Dihydrogen under certain reaction conditions, combines with almost all elements to form binary compounds, called hydrides except with few which are given below Choose one of the options
The lowest region of atmosphere in which the human being along with other organisms live is known as:
The range of most suitable indicator which should be used for titration of X - Na+ (0.1 M, 10 ml) with 0.1 M HCl should be ( Given : kb(X-) = 10-6 )
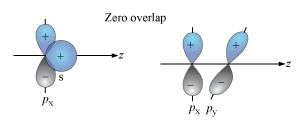
Which of the following orbitals give a zero overlap?
Which pair of elements from different groups resembles each other the most in their chemical properties?
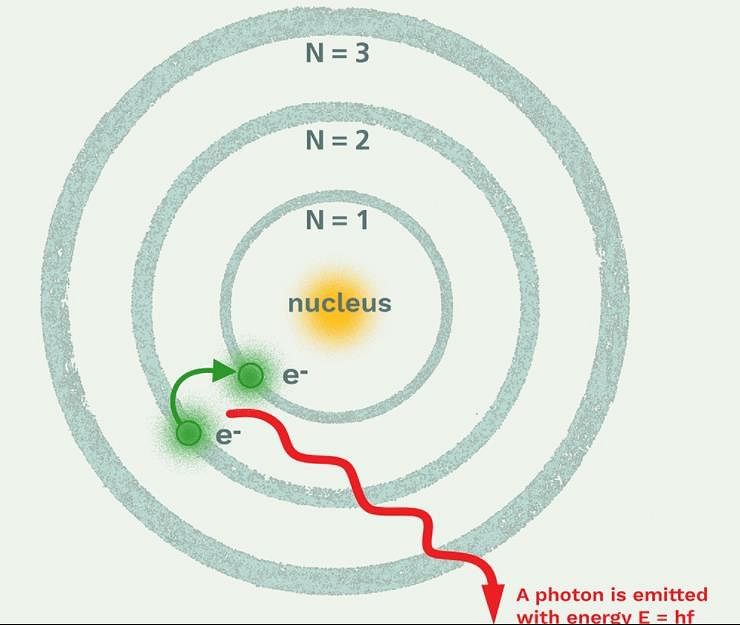
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?
Requirement of macronutrient per acre of the land is
Which type of a property is the Brownian movement of colloidal solution?
The rate law for the reaction 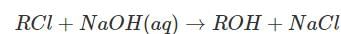 is given by rate = k[RCl]. The rate for this reaction
is given by rate = k[RCl]. The rate for this reaction
The maximum number of electrons that can have principal quantum number, n = 3 and spin quantum number,
Which of the following is true about chemisorption?
(Mily Cloud)
The chemical formulae of A and B are -?
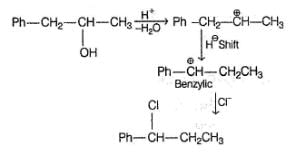
A chemist isolated a compound A with molecular formula C7H13Br. A undergoes very fast SN1 reaction. Spectroscopic evidence indicated that compound A has the following structural characteristics : It contains five sp3-hybridised carbon atoms. Among those five sp3 carbon atoms, three are methyl groups, one CH2 group and one CH group.
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
Q.
If the starting compound A is brominated in gas phase in the presence of a Lewis acid catalyst, a tribromide would result from addition of Br2 to . How many different structures of stereoisomers can be drawn for this tribromide?
Select the correct observation about electrolysis.
|
30 tests
|