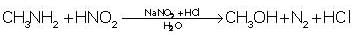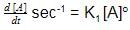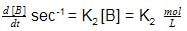UP PGT Chemistry Mock Test - 5 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 5
Which of the following amine liberates nitrogen gas on reaction with HNO2 ?
A fresh precipitate can be transformed into colloidal sol by:
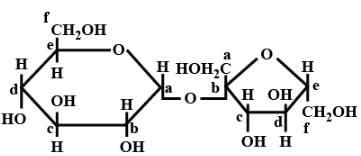
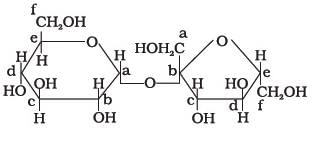
Structure of a disaccharide formed by glucose and fructose is given below. Identify anomeric carbon atoms in monosaccharide units.

Consider following two reactions
Units of k1 and k2 are expressed in terms of molarity (mol L–1) and time (sec–1) as –
[AIEEE-2002]
Which reagent given below does not produce any visible change when added to ethylene glycol?
Which one of the following is a water soluble vitamin?
How many distinct alkene products are possible when the alkyl iodide given below undergoes E2 elimination?
Which of the following acids does not exhibit optical isomerism?
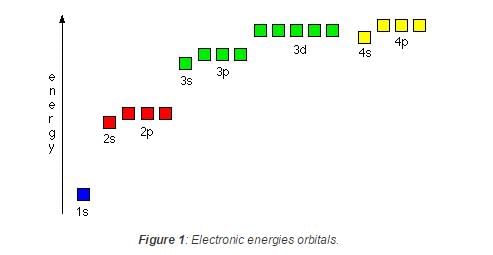
Which of the following sets of quantum numbers represents the highest energy of an atom ? [AIEEE 2007]
The reaction of SOCI2 on alkanols to form alkyl chlorides gives good yields because
What is the most efficient method to get water with zero degrees hardness?
Which has lowest and highest first ionisation enthalpy in 3d series?
Cellulose acetate (rayon) and cellulose nitrate are __________ polymers
The alkaline earth metals, which do not impart any colour to Bunsen flame are -
Catalytic converters must be used in cars to reduce the to reduce the effect of exhaust fumes on the atmosphere. The converter should contain one of the main components
Li shows difference in properties than rest of the members of its group but shows similarities with:
I− reduces IO3- and I2 and itself oxidised to I2 in acidic medium. Thus, final reaction is
Which one of the following is not a condensation polymer?
K2Cr2O7 is an oxidising agent in acidic medium. How many O-atoms are directly linked to each Cr-atom?
An ion M2+, forms the complexes [M(H2O)6]2+, [M(en)3]2+ and [MBr6]4-, match the complex with the appropriate colour.
For the equilibrium,
at 1000 K. If at equilibrium pCO = 10 then total pressure at equilibrium is
For the 115th element _________ is the name as per IUPAC nomenclature and __________ is the official name.
consider the following zonolysis reaction
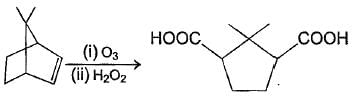
The correct statement about the above product formed is
Which of the following does not show optical isomerism?
In the following reaction, the polymer formed is ?

Which of the following is thermally the most stable?
PCl5 dissociation a closed container as :
PCl5(g) 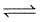 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is α, the partial pressure of PCl3 will be :
|
30 tests
|