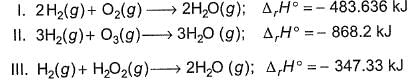UP PGT Chemistry Mock Test - 4 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 4
A gas absorbs 200 J of heat and expands by 500 cm3 against a constant pressure of 2 x 105 Nm-2. Change in internal energy is
The catalytic activity of transition metals and their compounds is mainly due to
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
An ethanol-water solution is prepared by dissolving 10.00 mL o f ethanol (C2H5OH, d = 0.789 g mL-1) in a sufficient volume o f w ater to produce 100 mL of solution with a density, d = 0.982 g mL-1. Match the concentration term in Column I with its value in Column II and select the answer from the code
Identify the incorrect statement among the following.
The type of hardness that occurs due to the presence of bicarbonate of calcium and magnesium ions hardness is _______________
The information that is/are needed to determine the molar mass of an unknown solute is/are
Which of the follownig compounds is (S)-4-chloro-1-methylcyclohexene ?
Nitro compounds are reduced by iron scrap and hydrochloric acid to yield one of the following compounds.
An aqueous solution of a solute AB has boiling point of 101.08° C and freezes at -1 .80 °C . AB is found to be 100% ionised at boiling point. If Kb /Kf = 0.3, then AB
Solution of sodium metal in liquid ammonia is a strong reducing agent due to presence of -?
Passing carbon dioxide through slaked lime gives:
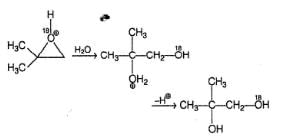
In the following hydrolysis reactio, the major final product is
The number of bond pairs and lone pairs in rhombic sulphur molecule are
Which of the following reactions will yield 2, 2 - dibromopropane?
[AIEEE 2007]
According to the VSEPR theory, the geometry and shape of the molecule depends upon:
what is incorrect regarding cis -1, 3-dibromo - trans-2,4-dichlorocyclobutane ?
Passage II
In hydrogen economy fuel-cell,anodic and cathodic reactions are
Anodic
H2+2OH- → 2H2O + 2e-
Cathodic
O2+2H2O + 2e- → 4OH-
67.2 L H2 at STP react in 15 min.Entire current is used for electro deposition of copper from copper(II) sulphate solution. Average current produced in fuel cell is
What is aggregation of colloidal particles into insoluble precipitate by addition of some suitable electrolyte called?
Consider the following oxidation/reduction process,
Q. Magnetic moment does not change in
A 300 mL solution of NaCl was electrolysed for 60.0 min. If the pH of the final solution was 12.24,average current used is
Which of the following element groups are considered types of metals?
Based on the following thermodynamic data,
Q. Which oxidising agent will generate the greatest amount of energy per gram of oxidising agent?
Oxidation numbers of P in PO4−3, of S in SO42− and that of Cr in Cr2O72− are respectively,

Suitable reagent for following cenversion will be :
10 dm3 of an ideal monoatomic gas at 27° C and 1.01 x 105 Nm-2 pressure are heated at constant pressure to 127°C. Thus entropy change is
Find the number of waves made by a Bohr’s electron in one complete revolution in its 3rd orbit
|
30 tests
|