UP PGT Chemistry Mock Test - 1 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 1
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The incorrect statement concerning E1 reaction is
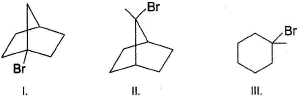
Which of the following alkyl halides is respectively most and least electrophilic in SN1 reaction?
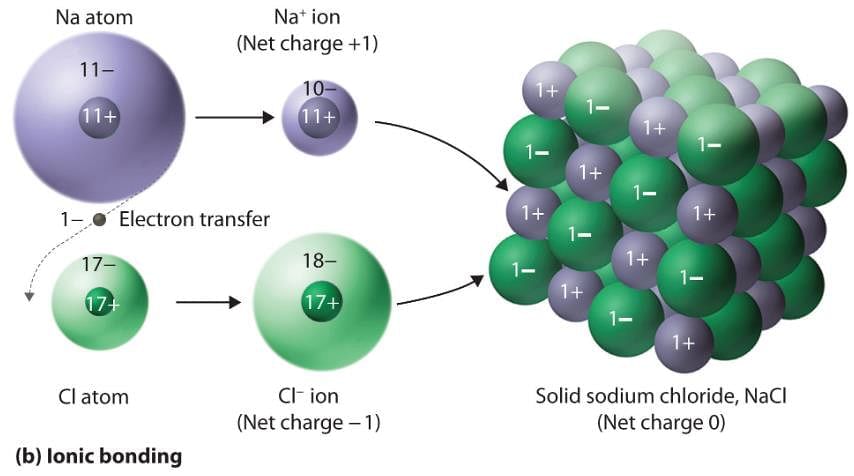
In which of the following solids, ions of opposite charges are held together by strong electrostatic forces of attraction?
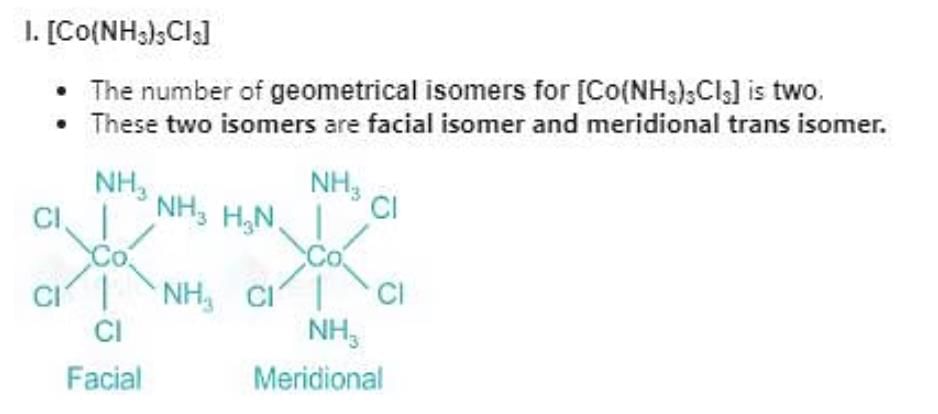
Which of the following compounds will show facial and meridional isomerism?
(A)[Co(NH3)3Cl3]
(B)[Co(acac)3]
(C)[Co(dien)(NO2)3]
(D)[Co(gly)3]
In the gas phase water is a bent molecule with a bond angle of
Which of the following concentration factor is affected by change in temperature ?
[AIEEE-2002]
Direction (Q. Nos. 1 - 6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
A pure enantiomer with molecular formula C6H13OBr, when reacted with PBr3, an achiral product C6H12Br2 is obtained that has no chiral carbon. The compound which satisfy this condition could be (no bond to chiral carbon is broken during the reaction)
One mole of N2O4(g) at 300 K is left in a closed container under one atm. It is heated to 600 K when 20% by mass of N2O4 (g) decomposes to NO2(g). The resultant pressure is :
How many atoms of Oxygen are there in 18g of water?
(Hint: Avogadro’s Number = 6.02 x 1023 atoms/mol)
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The equilibrium which is not affected by volume change at constant temperature is
Which of the following are not functional isomers of each other?
Which among the following substances is an example of multimolecular colloids?
In the earth’s atmosphere, hydrogen exists in the form of
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which is incorrect statement?
Which of the following is not the name of the 104th element?
Substances that are strongly attracted by applied magnetic field and can be permanently magnetized are
How many minimum no. of C-atoms are required for position & geometrical isomerism in alkene?
Solubility of CuS(l), ZnS (II) and Na2S (III) in water is in the order
Out of the following, amphiprotic species are
I : HPO32-
II OH-
III H2PO4-
IV HCO3-
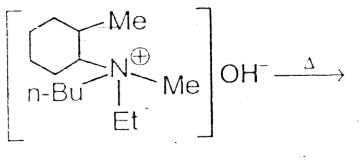
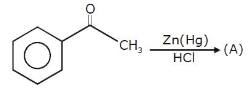
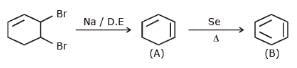
The alkene formed as a major product in the above elimination reaction is
[AIEEE 2006]
Which of the following tests can be used to distinguish between two isomeric ketones: 3- pentanone and 2- pentanone?
Which is the correct order w.r.t the given property?
On mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane. This alkane could be -
[AIEEE-2003]
|
30 tests
|