Indian Army Agniveer Technical Mock Test - 1 - Indian Army Agniveer MCQ
30 Questions MCQ Test - Indian Army Agniveer Technical Mock Test - 1
Droupadi Murmu, who recently became the first woman tribal President of India, belongs to which tribe?
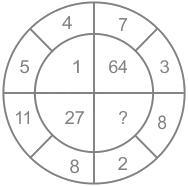
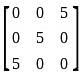
Assuming that the characters in the given figure follow similar pattern, find the missing number.
National motto of India “Satyameva jayate” means _______.
The Government of India releases MSP of agricultural produce from time to time. What is the full form of MSP?
A box provides Rs. 77 in the denominations of one rupee, 50 paise, and 25 paise coins. The number of 50 paise coins is double that of 25 paise coins and four times that of one rupee coins. How many 50 paise coins are there in box?
Suppose A1 , A2 , ..., A30 are thirty sets each having 5 elements and B1, B2, ..., Bn are n sets each with 3 elements, let 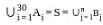 and each element of S belongs to exactly 10 of the Ai ’s and exactly 9 of the B's. then n is equal to
and each element of S belongs to exactly 10 of the Ai ’s and exactly 9 of the B's. then n is equal to
A sum of money becomes Rs 1500 in 3 years at a 2% simple rate of interest per annum, then in how many years will it become Rs. 1800?
If 2log(x+3) = log81 then the value of x.
The equation of the circle passing through the foci of the ellipse x216+y29=1, and having centre at (0, 3) is
Determine the value of sin21π2 and cos(-1740°)?
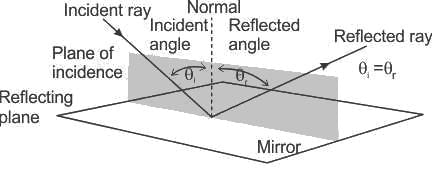
If the angle of incidence formed on a concave mirror at a point is 30° then the angle of reflection will be:
Which of the following is correct about reaction of phosphorus with water ?



 is a
is a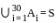
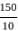 = 15
= 15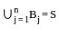
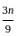 =
= 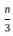
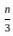 = 15
= 15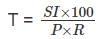
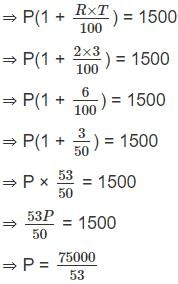
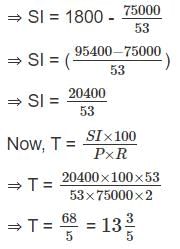
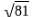
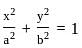 ; (a > b)
; (a > b)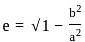
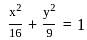
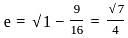
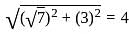
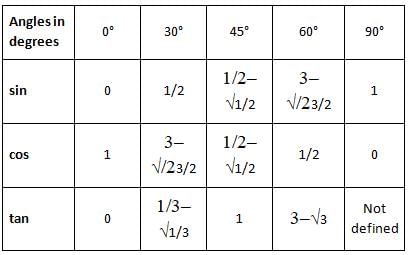
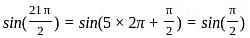
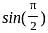 is 1.
is 1.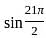 is 1 and the value of cos (-1740°) is 1/2.
is 1 and the value of cos (-1740°) is 1/2.