Indian Army Agniveer Technical Mock Test - 3 - Indian Army Agniveer MCQ
30 Questions MCQ Test - Indian Army Agniveer Technical Mock Test - 3
The 1972 Simla Agreement was signed between
Black soil, found in the Deccan Traps is considered highly suitable for the cultivation of ________ crops.
James Webb Space Telescope’s development was done by NASA in collaboration with which two Space agencies?
A series is given with one term missing. Select the correct alternative from the given ones that will complete the series.
AMRP, ERXW, IWDD, MBJK, ?
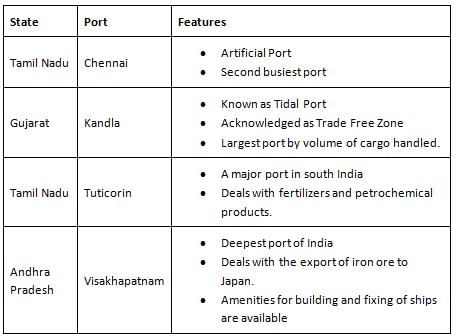
Which of the following is a tidal port?
Which of the following letter-clusters will replace the question mark (?) in the given series?
DBK, GDL, JFM, MHN, ?
Let {x} denotes the fractional part of a real number x. Then 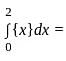
Solution of the differential equation cos x dy = y (sin x - y) dx, 0 < x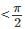 is
is
A train of length 850 m which travels at a speed of 162 kmph crosses a Tunnel in 63 seconds. Find the length of the Tunnel? (In metres)
Two persons are standing on either side of a 1200 m long bridge, if they walk towards each other at the speed of 5 m/min and 10 m/min respectively, in how much time will they meet?
If x = 2t and y = 2t2, then what is 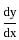 ?
?
For the function f(x) = x + 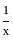 , x ∈ [1, 3], the value of c for mean value theorem is
, x ∈ [1, 3], the value of c for mean value theorem is
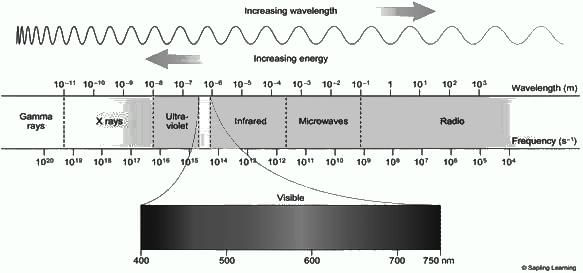
What is the approximate range of wavelength in case of visible lights?
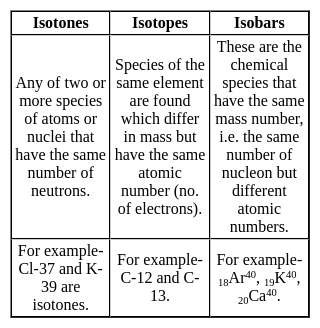
Which one among the following contains the most neutrons?
Which of the following is the isotone of 32Ge76



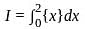
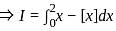
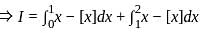
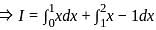
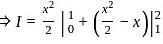
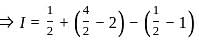
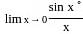 ?
?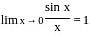
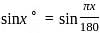
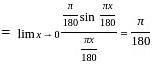
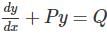 solve by following the steps
solve by following the steps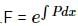
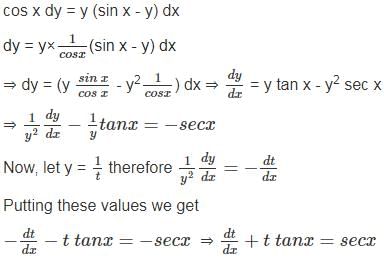

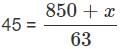
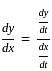 , where t is the parameter.
, where t is the parameter.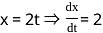 = 2
= 2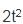
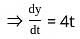 = 4t
= 4t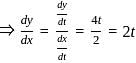
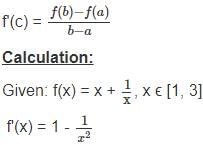
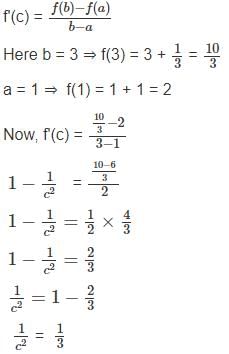

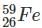
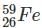
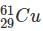
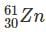
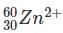
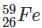 with 33 neutrons.
with 33 neutrons.