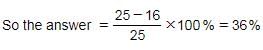Bihar STET Paper 2 Chemistry Mock Test - 7 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 7
Mole fraction of ethanol in ethanol-water solution is 0.25. Thus, this solution is
Amongst the following compounds, the optically active alkane having lowest molecular mass is -
[AIEEE-2004]
What is the major bromination product in the following reaction?
Consider the following pairs
Q. More stable species in each pair is
18 g of glucose (C6H12O6) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100º C is -
[AIEEE 2006]
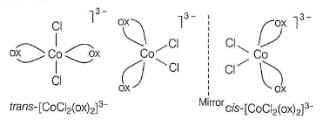
Which of the following will have three stereoisomeric form ?
i. [Cr(NO3)3(NH3)3]
ii. K3[Co(C2O4)3]
iii. K3[Co(C2O4)2CI2]
iv. [Co(en)2CIBr]
The major organic product formed in the following reaction is
How many structural isomers are possible with molecular formula C4H10O ?
What is true regarding a meso form of a compound?
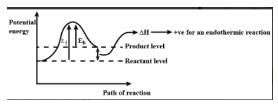
Consider an endothermic reaction X → Y with the activation energies Eb and Ef for the backward and forward reactions, respectively. In general
[AIEEE-2005]
(Yellow ppt) T X
Y. (Yellow ppt) + Z (pungent smellinggas)
If X gives green flame test. Then, X is :
Which method cannot be used for the coagulation of the lyophobic sol?
Different kinds of bonds and interaction present within CuSO4 • 5H2O. They can be
I. σ-bond
II. π-bond
III. coordinate bond
IV. electrostatic force of attraction
V. H-bond due to dipole-dipole interaction
VI. H-bond due to ion-dipole interaction
Select the correct types of bonds/interactions.
For moderation of the climate and body temperature of living beings, the responsible factor is:
Which of the following atom has been assigned only single oxidation number?
Maximum number of compounds are known in the case of which inert gas?
The treatment of CH3MgX with produces
[AIEEE 2008]
In which of the following reactions, increase in the pressure at constant temperature does not affect the moles at equilibrium.
A teacher can be satisfied that the students are able to grasp the concept delivered by him/her by
Mrs. Suman cannot score her students objectively. She is probably using
Which of the following tools/methods does National Education Policy 2020 propose for assessment of children?
(i) Role plays
(ii) Group work
(iii) Portfolios
(iv) Projects
Variability in learning styles of students :
Urban sewage discharge and industrial effluents are the main cause of
A student multiplied a number by 4/5 instead of 5/4. What is the percentage error in the calculation?
Select the related number from the given alternatives.
49 : 343 :: 121 : ?