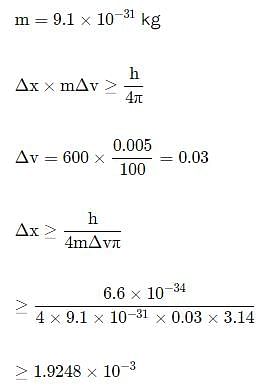KVS PGT Chemistry Mock Test - 8 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test KVS PGT Exam Mock Test Series 2024 - KVS PGT Chemistry Mock Test - 8
Directions: Improve the bracketed part of the sentence.
Q. The Finance Minister presented data on the large sums of money (deposit into) some bank accounts, causing problems in the economy.
Directions: In the following question, one part of the sentence may have error(s). Find out the part of the sentence having an error and select the appropriate option. If a sentence is free from error, select 'No error' as your answer.
Q. The rich man (1)/ killed him (2)/ and his own children (3)/ No error (4)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the systematic display of data along with graphics, sounds, movies, etc., called?
In which of the following National Education Policy the concept of socially useful productive work was introduced?
How does NEP 2020 propose to address the flexibility in subject choices for students in higher education?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
A handbook gives the concentration of water in a 2.772 M aqueous solution of NaOH as 998.0 g L-1.
Q. Molality of solution is
Which of the following complex species is not expected to exhibit optical isomerism?
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of
Electron-rich hydrides has excess electrons that are present as
In which of the following pairs both the ions are coloured in aqueous solution? (Atomic number, Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
In an atom, an electron is moving with a speed of 600m/s with an accuracy of 0.005%. Certainity with which the position of the electron can be located is (h = 6.6 ×10−34 Js)
Consider the following compounds
I. K4[Fe(CN)6]
II. NH4Cl
III. H2SO4
IV. [Ni (CO)4]
Q.
Ionic, covalent and coordinate bonds are present in
An electrochemical cell was based on the following reaction:
Mn(OH)2(s) + H2O2(aq) → MnO2(s) + 2H2O (e)
During the opeartion of this for 1 min, 0.135 g of MnO2 was produced. What is the average electric current (in ampere ) produced by the cell?
In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (Aand B) are doubled, rate law for the reaction can be written as
Which of the following is nonpolar but contains polar bonds?
Azide ion exhibits an (N—N) bond order of 2 and may be represented by resonance structures I, II and III given below :
Select the correct statement(s) about more contributions,