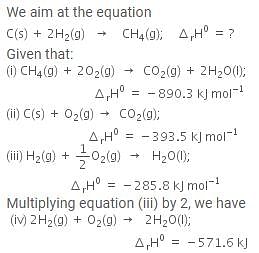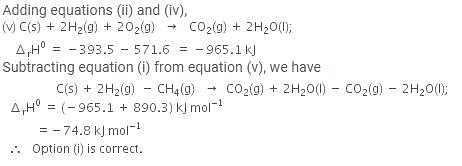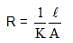KVS PGT Chemistry Mock Test - 3 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test KVS PGT Exam Mock Test Series 2024 - KVS PGT Chemistry Mock Test - 3
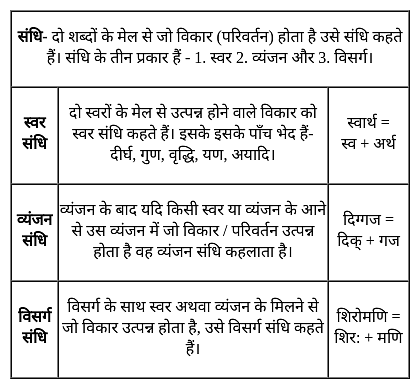
नीचे दिए गए शब्दों का सही संधि वाला विकल्प पहचानिए?
धर्म + अर्थ
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Who determines the minimum support price in India?
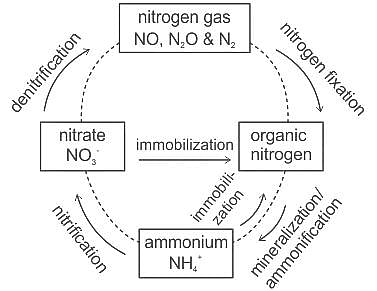
The process of converting ammonia into nitrates is called:
Which of the following compounds will exhibit cis-trans isomerism?
Passage I
A constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.
Thus Cl2 formed under STP condition is
In which of the following, is hydrogen peroxide not stored?
The enthalpy of combustion of methane, graphite and dihydrogen at 298 K are - 890.3 kJ mol-1, - 393.5 kJ mol-1 and - 285.8 kJ mol-1 respectively. Enthalpy of formation of CH4(g) will be
Primary alcohol can easily be prepared from primary alkyl halide via SN2 reaction with aqueous NaOH. However, similar method does not work for the preparation of tertiary alcohol. Which reaction can be used for the efficient preparation of tertiary alcohol {tertiary butanol) from tertiary butyl bromide?
In the modern periodic table, which period contains 32 elements?
The metal that can not be obtained by electrolysis of an aqueous solution of its salt is
[JEE Main 2014]
At 87°C, the following equilibrium is established
H2(g) + S(s)  H2S(g) Kp = 7 × 10-2
H2S(g) Kp = 7 × 10-2
If 0.50 mole of hydrogen and 1.0 mole of sulfur are heated to 87°C in 1.0 L vessel, what will be the partial pressure of H2S at equilibrium ?
If Ksp for HgSO4 is 6.4 × 10-5, then solubility of this substance in mole per m3 is
Pick out the most reactive alkyl halide for an SN1 reaction.
Phenyl magnesium bromide reacts with methanol to give -
[AIEEE 2006]
The principle involved in paper chromatography is:
The correct order of in creasing order of radiiions Br- , F-, O2- and S2- is as follow
In the third period of the periodic table the element having smallest size is
Glucose solution is one molal. Glucose present in 1 kg glucose solution is
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?
A resistance of 50Ω is registered when two electrodes are suspended into a beaker containing a dilute solution of a strong electrolyte such that exactly half of the them are submerged into solution. If the solution is diluted by adding pure water (negligible conductivity) so as to just completely submerge the electrodes, the new resistance offered by the solution would be
1 c.c. of 0.1N HCl is added to 99 CC solution of NaCl. The pH of the resulting solution will be