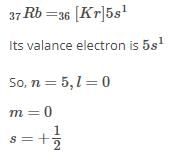AWES PGT Chemistry Mock Test - 6 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2025 - AWES PGT Chemistry Mock Test - 6
What is the Gross Merchandise Value (GMV) achieved by the Government e-Marketplace (GeM)?
Where was the 44th All India Criminology Conference of the National Forensic Science University (NFSU) held?
Who won the gold medal in the 50m rifle 3P event at the 2023 Asian Shooting Championship?
What is the name of the recently launched brand of National Cooperative Organics Ltd (NCOL)?
A complex compound in which the oxidation number of a metal is zero is
Which of the following correctly describe the relative nucleophilicities of methoxide and tertiary butoxide ion?
The metabolism of hormones in human body is an example of
Which of the following, do you think are the synthetic resins present in removal of permanent hardness?
Which of the alkyl chlorides listed below undergoes dehydrohaiogenation in the presence of a strong base to give 2-pentene as the only alkene product?
Among the following sets, highest boiling points are of the species.
I. HF, HCI, HBr, HI
II. H2O, H2S, H2Se, H2Te
III. NH3,PH3, AsH3,SbH3
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is
[JEE Main 2013]
Chemical species present in the environment are either naturally occurring or generated by human activities. Their interrelation with the surroundings is called:
The constant k used in rate equation is known as
The frequency of light emitted for the transition n = 4 to n = 2 of He+ is equal to the transition in H atom corresponding to which of the following
In a hypothetical solid C atoms are found to form cubical close packed lattice. A atoms occupy all tetrahedral vioids & B atoms occupy all octahedral voids. A and B atoms are of appropriate size, so that there is no distortion in CCP lattice of C atoms. Now if a plane as shown in the following figure is cut.Then the cross section of this plane will look like.
Electron affinity reflects the ability of an atom to accept an electron. Which is true of the alkaline earths?
90% of hydrogen peroxide is used as fuel in ______________
During an electrolysis of conc. H2SO4, perdissulphuric acid (H2S2O8) and O2 from in equimolar amount. The amount of H2 that will form simultaneously will be (2H2SO4 → H2S2O8 + 2H+ + 2e-)
In the following reactions except in one, oxygen is the reducing agent. Exceptional reaction is
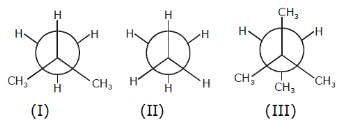
Correct order of mono bromination of given compound is :
Direction (Q. Nos. 18 and 19) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. S2O32- has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
How many valence electrons does a carbon atom have?
Which of the following heptanols are chiral 1-heptanol, 2-heptanol, 3-heptanol, 4-heptanol.
Among the following species octahedral shape is found in:
6.02 x1020 molecules of urea are present in 100 mL of its solution. The concentration of urea solution is (N0 = 6.02 x 1023 mol-1)
[AIEEE 2004]
A solution was made by dissolving 2 g of a solute in 100 g of acetone. The solution boiled at 56.95° C. The boiling point of pure acetone is 55.95° C, and the Kb =1.71°C/m. What is the molecular weight of the solute?
|
12 docs|30 tests
|