AWES TGT Chemistry Mock Test - 2 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 2
Barsana Biogas Project, which was seen in the news, is situated in which state?
Mataberi Pera & Pachra, recently got a GI tag, belongs to which state?
Who has been elected as the new Chairman of the Automotive Tyre Manufacturers' Association (ATMA)?
Rafah border crossing is located between which of the following?
Which country has declared a health emergency due to escalating cases of dengue fever in 2024?
When is the International Day to Protect Education from Attack?
In order to help a mentally challenged child in your class, which of the following strategies would you adopt?
Radius of Bohr’s orbit of H-atom is 52.9 pm. An emission in H-atom starts from the orbit having radius 1.3225 nm and ends at 211.6 pm.
Q. Spectral line appears in .......... region.
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X is
At what minimum atomic number, a transition from n = 2 to n = 1 energy level results in the emission of X-rays with wavelength 3.0 x 10-8 m?
In oxygen difluoride (OF2) and dioxygen difluoride(O2F2), the oxygen is assigned an oxidation number of
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The correct statement regarding a chiral compound is
For which of the following reaction the units of rate constant and rate of the reaction are same?
Which has lower standard reduction potential (SRP) value?
When body temperature is high, doctors advice consumption of light food. This is because
How many grams of Mgl2 must be added to 250 mL of 0.0876 M Kl to produce a solution with [l-] = 0.10 M?
Once the equilibrium is reached under given condition:
Measure of the amount of organic material in the water, in terms of how much oxygen will be required to break it down biologically is termed as
For the following gases equilibrium. N2O4(g) 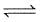 2NO2(g) Kp is found to be equal to Kc. This is attained when temperature is
2NO2(g) Kp is found to be equal to Kc. This is attained when temperature is
Three cell A, B and C has equilibrium constant in the ratio 1:4 : 9 respectively. Arrange the following cells in the order of increasing Gibbs free energy.
The combination of a carbonyl group and a hydroxyl group on the same carbon atom is called a __________ group.
0.004 M Na2S04 aqueous solution is isotonic with 0.01 M glucose solution at 300 K. Thus, degree of dissociation of Na2S04 is
Passage II
Consider the following structure
Q. Shortest (C— H) bond is
Direction (Q. Nos. 21-25) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Passage I
Electronegativity values (EN) of elements have been given (in Pauling’s scale)
Graph shows variation of percentage ionic character with (EN) difference of two elements.
Q. Which of the following bonds is most polar?
The bond enthalpy of ___________ molecule is 435.8 kJ mol-1.
The basic character of the transition metal monoxides follows the order
(Atomic Number, Ti = 22, V = 23, Cr = 24, Fe = 26)
For a multi-electron atom, set of quantum numbers is given as
2,0,0,1/2 ; 2,0,0,-1/2
Q. Thus, the next higher allowed set of n and / quantum numbers for this atom in its ground state is
The correct IUPAC name of the folllowing compound is














