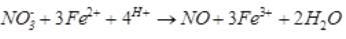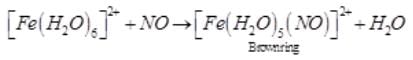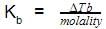AWES TGT Chemistry Mock Test - 9 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 9
Which company has partnered with Adani Total Energies to set up EV charging infrastructure?
India signed a Memorandum of Understanding with which country for sharing open-sourced digital public infrastructure?
Which bank secured seven awards at the 19th Banking Tech Conference?
According to the Organisation for Economic Co-operation and Development (OECD), which country emerges as the foremost aid provider in 2022?
Which country has officially announced the permanent closure of its embassy in India, citing persistent challenges from the Indian government as the primary reason?
Kritika who does not talk much at home talks a lot at school. It shows that:
The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals, the lowest solubility of LiF in water is due to
The equilibrium constant Kc for the reaction,
A(g) + 2B(g)  3C(g) is 2 × 10-3
3C(g) is 2 × 10-3
What would be the equilibrium partial pressure of gas C if initial pressure of gas A & B are 1 & 2 atm respectively.
A brown ring is formed in the ring test for NO3– ion. It is due to the formation of
The compound which gives red colour with Fehling’s solution?
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
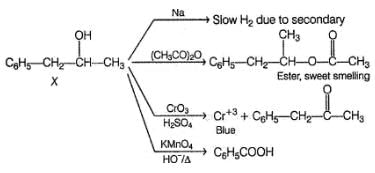
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
The structure of X is
Number of electrons having l + m value equal to zero in 26Fe may be
Consider the cell Ag(s)|AgBr(s)|Br-(aq)||AgCl(s)|Cl-(aq)|Ag(s) at 25°C. The solubility product constants of AgBr & AgCl are respectively 5 X 10 – 13 & 1 X 10 – 10. For what ratio of the concentrations of Br- & Cl- ions would the emf of the cell be zero ?
The factors which are responsible for the stability of lyophilic sols are:
The molal elevation constant is the ratio of the elevation in B.P to:
NO2 can be represented as
Q. Formal charge on each oxygen atom is
Statement-1: In electrochemical cell, we cannot use KCI in the salt bridge if anodic or cathodic compartment consists of Ag+ of Pb2+ ion.
Statement-2: Salt bridge is employed to maintain the electrical neutrality and to minimize the liquid-liquid junction potential.
Pick out the strongest substrate(s) for a SN1 reaction.
In which group of the modern periodic table are halogens placed?
Aqueous solution of barium phosphate which is 100% ionised has ΔTf / Kf as 0.40. Hence, given solution is x * 10-2 molal. What is the value of x?
Passage l
Sulphur undergoes a phase transition between 80 and 110°C
S(rhombic) S (monoclinic); ΔH° = 3.213 kJ mol-1; ΔS° = 8.71 JK-1 mol-1
Q. Temperature at which ΔG° = 0, is
Which of the following is not a use of hydrogen peroxide?
Methyl cyclohexane react with Br2 in presence of u.v. light major product will be
Oxidation of ammonia with CuO produce a gaseous chemical which can also be obtained by:
Osmotic pressure of 40% (wt./vol.) urea solution is 1.64 atm and that of 3.42% (wt./vol.) cane sugar is 2.46 atm. When equal volumes of the above two solutions are mixed, the osmotic pressure of the resulting solution is -
Name the element which forms the stable fluoro complex anions: