NEET Exam > NEET Tests > Chemistry Class 12 > Test: Study of Nitrogen Functional Group - NEET MCQ
Test: Study of Nitrogen Functional Group - NEET MCQ
Test Description
20 Questions MCQ Test Chemistry Class 12 - Test: Study of Nitrogen Functional Group
Test: Study of Nitrogen Functional Group for NEET 2025 is part of Chemistry Class 12 preparation. The Test: Study of Nitrogen Functional Group questions and answers have been
prepared according to the NEET exam syllabus.The Test: Study of Nitrogen Functional Group MCQs are made for NEET 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Study of Nitrogen Functional Group below.
Solutions of Test: Study of Nitrogen Functional Group questions in English are available as part of our Chemistry Class 12 for NEET & Test: Study of Nitrogen Functional Group solutions in
Hindi for Chemistry Class 12 course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Study of Nitrogen Functional Group | 20 questions in 30 minutes | Mock test for NEET preparation | Free important questions MCQ to study Chemistry Class 12 for NEET Exam | Download free PDF with solutions
Test: Study of Nitrogen Functional Group - Question 1
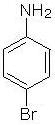
What is the common name of the given compound?
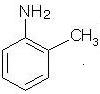

Detailed Solution for Test: Study of Nitrogen Functional Group - Question 1
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 2
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 3
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 4
Test: Study of Nitrogen Functional Group - Question 5
Reduction of alkanenitriles with sodium and alcohol or LiAlH4 is called:
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 5
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 6
Test: Study of Nitrogen Functional Group - Question 7
The carbylamine reaction is given by compounds having:
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 7
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 8
Test: Study of Nitrogen Functional Group - Question 9
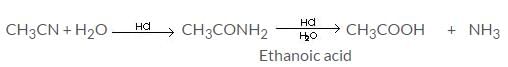
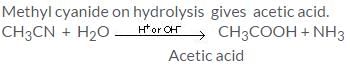
Hydrolysis of methyl cyanides with dil. mineral acids gives:
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 9
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 10
Test: Study of Nitrogen Functional Group - Question 11
Identify the most basic compound from the following.
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 11
Test: Study of Nitrogen Functional Group - Question 12
Amines that have one of their three hydrogen atoms replaced by an alkyl or aromatic substituent are referred to as
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 12
Test: Study of Nitrogen Functional Group - Question 13
Write the IUPAC name of the given compound.
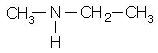
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 13
Test: Study of Nitrogen Functional Group - Question 14
Pyridine is less basic than triethylamine because:
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 14
Test: Study of Nitrogen Functional Group - Question 15
If two hydrogen atoms of ammonia are replaced by alkyl group then the amine is:
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 15
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 16
Test: Study of Nitrogen Functional Group - Question 17
Benzonitrile is the IUPAC name of the compound:
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 17
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 18
Test: Study of Nitrogen Functional Group - Question 19
In amines ,the hybridisation state of N is:
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 19
Detailed Solution for Test: Study of Nitrogen Functional Group - Question 20
|
75 videos|339 docs|78 tests
|
Information about Test: Study of Nitrogen Functional Group Page
In this test you can find the Exam questions for Test: Study of Nitrogen Functional Group solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Study of Nitrogen Functional Group, EduRev gives you an ample number of Online tests for practice



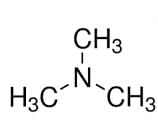 is a tertiary amine with IUPAC name:
is a tertiary amine with IUPAC name: