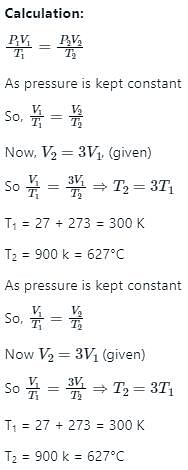Test: Thermodynamics- 1 - Mechanical Engineering MCQ
25 Questions MCQ Test Mechanical Engineering SSC JE (Technical) - Test: Thermodynamics- 1
Work and heat are
1. Point functions
2. Path functions
3. Exact Differential
4. Inexact Differential
1. Point functions
2. Path functions
3. Exact Differential
4. Inexact Differential
Which of the following are pure substance.
i.O2 & N2 mixture
ii. Moist Air
iii. Dry Air.
When water is at saturated condition (i.e.Saturated liquid or saturated vapour) no. of independent intensive properties required to determine the state is
The steam point is 100º C or 212F. The Ice point is 0ºC or 32ºF.The relationship b/w ºC and ºF is
It E = Energy Transfer M = Mass transfer In a isolated system which of the following is true,
An ideal gas at 27°C is heated at constant pressure till the volume becomes three times. The final temperature of the gas will be:
In a new temperature scale say of the boiling and freezing point of water at one atmosphere are 100º f and 300º f respectively, corelate this scale with centrigate scale. The reading of 0º f on the centrigate scale is
The pressure and temperature of tripple point of water is :
The diagram below shows a compression process in P-V diagram. Work required by a centrifugal compressor is equal to the area
A container contains mixure of O2 gas and N2 gas. The number of independent intrinsic variables required to completely define The state of the system is
Change in internal energy in a reversible process occuring in a closed system is equal to the heat transfered in the process occurs at constant
A water heater of power 2kw is switched on for 10 minutes. The capacity of heater is 10 liter of water . If heat capacity of water is 4 KJ/KgK.The rise in temperature is :
In a isothermal process the fluid expands from 5 bar and 2m3 to 2.5 bar and 4m3. The heat added during the process is 50 kJ. The net work done during the process is.
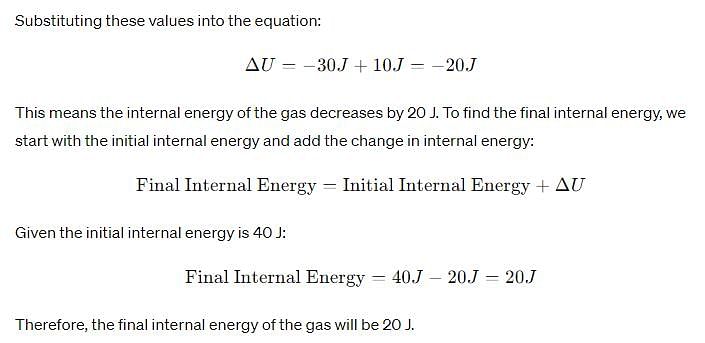
In a thermal process, the pressure of a fixed mass of a gas is changed in such a manner that the gas molecules give out the heat of 30 J and work of 10 J is done on the gas. If the initial internal energy of the gas was 40 J, then the final internal energy will be?
A real gas obeys perfect gas laws at very.
1. Low temperatures
2. high temperatures
3. Low pressures
4. High pressures
A heat engine works on carnot cycle work output is half of heat rejected to sink. The efficiency of the engine is
A Carnot cycle works between 27 ºC and 327 ºC. If engine produces 100 kJ of work the entropy change during heat rejection is .
1. An adiabatic process is always a constant entropy process
2. In an adiabatic process heat transfer is equal to zero.
|
5 videos|103 docs|59 tests
|