MP Police SI (Technical) Mock Test - 4 - MP Police SI MCQ
30 Questions MCQ Test - MP Police SI (Technical) Mock Test - 4
The pair of compounds which cannot exist together in solution is:
The molecule of a monatomic gas has only three translational degrees of freedom. Thus, the average energy of a molecule at temperature ' 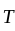 ' is ______.
' is ______.
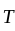 ' is ______.
' is ______.A solid disc and a solid sphere have the same mass and same radius. Which one has the higher moment of inertia about its centre of mass?
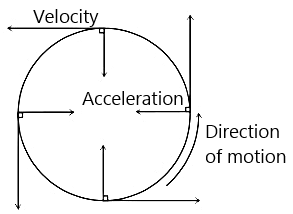
The circular motion of a particle with constant speed is:
The correct order of reactivity of the following metals is:
On increasing the length of wire by four times and increasing the radius of wire by 'X' times the resistance of wire remains unchanged then calculate the value of 'X'.
An object is dropped from a height of 500 m. How long will it take to reach the floor (in seconds)? (use g = 10 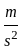 ).
).
Choose the incorrect statement from the following.
The raw materials used for the manufacture of Portland cement are
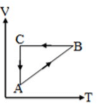
The cyclic process  shown in the
shown in the  diagram is displayed with the constant mass of an ideal gas. Below given diagram is the same as:
diagram is displayed with the constant mass of an ideal gas. Below given diagram is the same as:
At what temperature (in degree celsius), the numerical values on Celsius and Fahrenheit scales become equal?
A particle is moving along a circular track of radius  with a uniform speed. The ratio of the distance covered and the displacement in half revolution is:
with a uniform speed. The ratio of the distance covered and the displacement in half revolution is:
After completing the gold foil experiment, Rutherford concluded that the size of the nucleus is very small compared to the size of the atom because:
A point is selected at random inside a rectangle and perpendiculars are drawn on each side from the point. The sum of these perpendiculars is 24 cm. If the length of the rectangle is 3 times the width, the perimeter of the rectangle will be:
The enormous diversity of protein molecules is due mainly to the diversity of:
If we plot a graph for  (voltage across resistor) vs current
(voltage across resistor) vs current 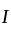 passing through a resistor, the slope of this graph represents:
passing through a resistor, the slope of this graph represents:
Which one of the following minerals is used as a fuel in nuclear power stations?
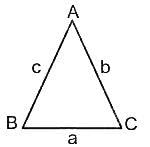
Find the sum of the cosine of the three angles of a triangle with sides 11 cm, 60 cm, and 61 cm.
What happens when a gas expands adiabatically?
The temperature at which a liquid changes into vapor after receiving heat is called :
 at room temperature?
at room temperature?If temperature of a room is 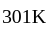 . The temperature in Celsius is:
. The temperature in Celsius is:
In the pressure cooker, the cooking is faster because the increase of vapour pressure:
The total internal energy of a mole of a monoatomic gas is equal to?
Kirchoff's first rule of any junction of several circuit elements in an electric circuit is based on the law of conservation of:
The pair of compounds having metals in their highest oxidation state is:


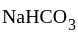 and
and 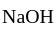 compounds cannot exist together in solution as:
compounds cannot exist together in solution as: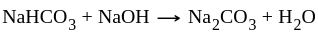
 Neutral salt
Neutral salt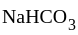 is a slightly acidic compound, while
is a slightly acidic compound, while 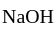 is a base. H-atom of
is a base. H-atom of 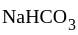 replace by base, i.e.,
replace by base, i.e., 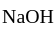 , give
, give 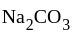 and water.
and water.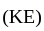 of translation per molecules of the gas is related to temperature by the relationship:
of translation per molecules of the gas is related to temperature by the relationship: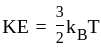 (degree of freedom of a monoatomic gas
(degree of freedom of a monoatomic gas 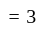 )
)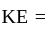 kinetic energy,
kinetic energy, 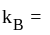 Boltzmann constant and
Boltzmann constant and 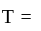 temperature.
temperature.
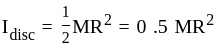
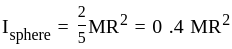
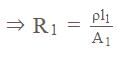
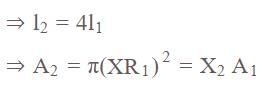
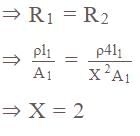
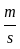
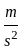
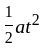
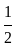
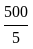
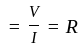 .
.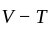 diagram, the
diagram, the 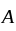 to
to 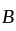 process is an isobaric spread, which is only in option (A). From
process is an isobaric spread, which is only in option (A). From 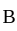 to
to 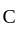 the temperature is synchronous with a decrease in temperature and pressure.
the temperature is synchronous with a decrease in temperature and pressure. 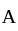 from
from 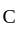 is an isometric compression.
is an isometric compression.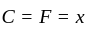
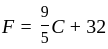
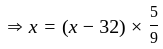
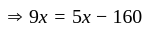
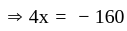
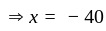
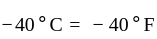
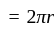
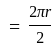
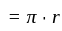
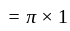 m
m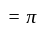 m
m Distance travelled
Distance travelled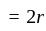
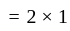 m
m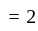 m
m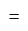 Displacement
Displacement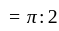
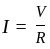
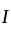 is the current through the conductor,
is the current through the conductor, 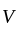 is the voltage across the conductor and
is the voltage across the conductor and 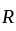 is the resistance of the conductor.
is the resistance of the conductor.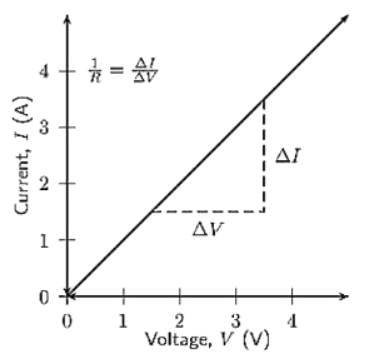
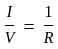
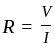
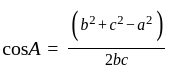
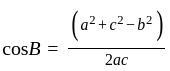
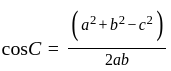
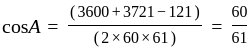
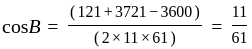
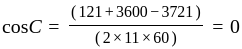
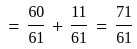
 -
-  bond due to the resonance effect of
bond due to the resonance effect of 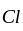 which has lone pair of electrons.
which has lone pair of electrons.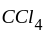 and
and 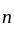 -hexyl chloride have covalent bonds, thus, cannot be broken.
-hexyl chloride have covalent bonds, thus, cannot be broken. 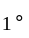 halide), the
halide), the 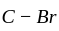 bond breaks easily as the carbocation formed is resonance stabilised and thus, it reacts with alc.
bond breaks easily as the carbocation formed is resonance stabilised and thus, it reacts with alc.  at room temperature and brown precipitates of
at room temperature and brown precipitates of 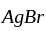 are formed.
are formed.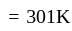
 Temperature in
Temperature in 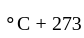
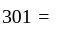 Temp
Temp 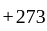
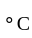
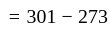

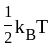
 degrees of freedom is given by
degrees of freedom is given by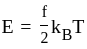
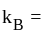 Boltzmann constant, and
Boltzmann constant, and 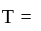 temperature,
temperature, 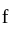 =degrees of freedom
=degrees of freedom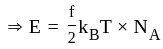
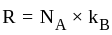 applying this in the above equation
applying this in the above equation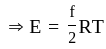
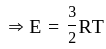 (As degree of freedom of monoatomic gas is
(As degree of freedom of monoatomic gas is 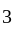 )
)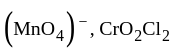 oxidation states of
oxidation states of 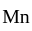 and
and 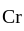 are +7 and +6 respectively.
are +7 and +6 respectively.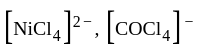 oxidation states of Ni and Co are +2 and +3 respectively.
oxidation states of Ni and Co are +2 and +3 respectively.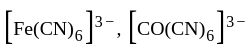 oxidation states of Fe and Co are +3 and +3 respectively.
oxidation states of Fe and Co are +3 and +3 respectively.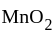 and
and 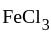 oxidation states of Mn and Fe are +4 and +3 respectively.
oxidation states of Mn and Fe are +4 and +3 respectively.










