Organic Chemistry MCQ - 1 (Advanced) - JEE MCQ
28 Questions MCQ Test - Organic Chemistry MCQ - 1 (Advanced)
Which of (a)-(d) is the correct IUPAC name of the following compound?
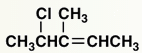
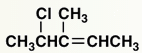
Which of the following statements would be true about this compound:
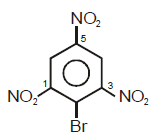
Ease of ionization to produce carbocation and bromide ion under the treatment of 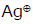 will be maximum
will be maximum
in which of the following compounds ?
in which of the following compounds ?
Ease of ionization to produce carbocation and bromide ion under the treatment of will be maximum
in whichof the following compounds ?
Which one of the following statements is True:
(1)
(2 )
Correct order of rate of hydrolysis or rate of reaction toward AgNO3 for following compounds is
Arrange the given phenols in their decreasing order of acidity:
Select the correct answer from the given code:
Which one of the following is the most acidic?
The correct pKa order of the follwoing acids is :
Arrange pH of the given compounds in decreasing order:
(1) Phenol
(2) Ethyl alcohol
(3) Formic acid
(4) Benzoic acid
Arrange acidity of given compounds in decreasing order:
Which of the above compounds reacts with NaHCO3 giving CO2
Identify electron - withdrawing groups in resonance among the following:
Identify electron - donating groups in resonance among the following:
In which of the following lone-pair indicated is involved in resonance :
In which of the following lone-pair indicated is not involved in resonance :
Identify the correct statement which is related to aromatic hydrocarbon?
Identify electron-donating groups in resonance among the following:
Identify electron - withdrawing groups in resonance among the following:
Which of the following reactions give aromatic compound ?



















