Olympiad Test: Materials: Metals & Non Metals - UPSC MCQ
20 Questions MCQ Test General Science(Prelims) by IRS Divey Sethi - Olympiad Test: Materials: Metals & Non Metals
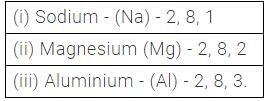
How many electrons are generally present in metals in their valence shell?
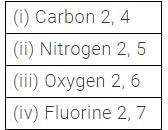
How many electrons are present in non- metals in their outermost shell?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
To which category do antimony and arsenic belong?
Identify the metal that replaces magnesium from its salt.
Aluminium foil is used for wrapping food. On which property is it used?
Identify the non-metal which exhibits yellow colour.
Which of the following are the properties of non-metals?
(i) They have low densities
(ii) They have low melting points
(iii) They are poor conductors of electricity
Which of the following non-metals exists in liquid state at room temperature?
Identify the metal that does NOT react with HCl
Which metal reacts vigorously with HCl to produce salt and hydrogen?
When non-metals combine with oxygen, what do they produce?
Identify the oxide of a non-metal which is neutral.
Phosphorus combines with oxygen to form oxides. How many types of oxides are formed by it?
Which of the following dissolves in water to form sulphuric acid?
Which of the following compounds is used to make photographic films?
Which material is used for making crucibles?
Which non-metal is used in the treatment of rubber during the process of vulcani-sation?
Which of the following is called a noble metal?
Why is tungsten used as a filament in electric bulbs?
|
39 videos|110 docs|262 tests
|
|
39 videos|110 docs|262 tests
|