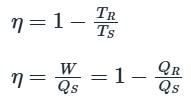Test: Second Law of Thermodynamics, Entropy - 2 - Mechanical Engineering MCQ
30 Questions MCQ Test GATE Mechanical (ME) Mock Test Series 2025 - Test: Second Law of Thermodynamics, Entropy - 2
Assigning the basic dimensions to mass, length, time and temperature respectively as M, L, T and θ (Temperature), what are the dimensions of entropy?
When a system reaches the state of equilibrium, the following property assumes its maximum value
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Assertion (A): If a graph is plotted for absolute temperature as a function of entropy, the area under the curve would give the amount of heat supplied.
Reason (R): Entropy represents the maximum fraction of work obtainable from heat per degree drop in temperature.
When a process becomes irreversible due to heat interaction between system and surrounding at the boundary due to finite temperature gradient, then the irreversibility is______
Consider the following statements:
In an irreversible process
1. Entropy always increases.
2. The sum of the entropy of all the bodies taking part in a process always increases.
3. Once created, entropy cannot be destroyed.
Of these statements which of the following options are correct ?
A system of 100 kg mass undergoes a process in which its specific entropy increases from 0.3 kJ/kg-K to 0.4 kJ/kg-K. At the same time, the entropy of the surroundings decreases from 80 kJ/K to 75 kJ/K. The process is:
The entropy of a mixture of ideal gases is the sum of the entropies of constituents evaluated at:
One kg of air is subjected to the following processes:
1. Air expands isothermally from 6 bar to 3 bar.
2. Air is compressed to half the volume at constant pressure
3. Heat is supplied to air at constant volume till the pressure becomes three fold
In which of the above processes, the change in entropy will be positive?
In which one of the following situations the entropy change will be negative
Increase in entropy of a system represents
Heat flows between two reservoirs having temperatures 1000 K and 500 K, respectively. If the entropy change of the cold reservoir is 10 kJ/K, then what is the entropy change for the hot reservoir?
Which one of the following pairs best expresses a relationship similar to that expressed in the pair “pressure-volume” for a thermodynamic system undergoing a process?
In the T-S diagram shown in the figure, which one of the following is represented by the area under the curve?
If a system undergoes an irreversible adiabatic process, then (symbols have usual meanings)
Which one of the following statements is not correct?
1600 kJ of energy is transferred from a heat reservoir at 800 K to another heat reservoir at 400 K. The amount of entropy generated during the process would be:
M1 kg of water at T1 is isobarically and adiabatically mixed with M2 kg of water at T2 (T1 > T2). The entropy change of the universe is:
Nitrogen gas (molecular weight 28) is enclosed in a cylinder by a piston, at the initial condition of 2 bar, 298 K and 1 m3. In a particular process, the gas slowly expands under isothermal condition, until the volume becomes 2m3. Heat exchange occurs with the atmosphere at 298 K during this process.
Q.
The work interaction for the Nitrogen gas is:
If a closed system is undergoing an irreversible process, the entropy of the system
In an experimental set-up, air flows between two stations P and Q adiabatically. The direction of flow depends on the pressure and temperature conditions maintained at P and Q. The conditions at station P are 150 kPa and 350 K. The temperature at station Q is 300 K.
The following are the properties and relations pertaining to air:
Specific heat at constant pressure, cp = 1.005 kJ/kg K;
Specific heat at constant volume, cv = 0.718 kJ/kg K;
Characteristic gas constant, R = 0.287 kJ/kg K.
Enthalpy, h = cpT
Internal energy, u = cvT
Q.
If the pressure at station Q is 50 kPa, the change in entropy in kJ/kg K is
Which point is taken as the standard reference point to define the Kelvin temperature scale?
A Carnot engine operates between 327°C and 27°C. If the engine produces 300 kJ of work, what is the entropy change during heat addition?
A cyclic process ABCD shown in the V-T diagram performed with a constant mass of an ideal gas. The process of p-V diagram will be as shown in
Assertion (A): Second law of thermodynamics is called the law of degradation of energy.
Reason (R): Energy does not degrade each time it flows through a finite temperature difference.
Consider the following statements:
1. Slow heating of water from an electric heater.
2. Isentropic expansion of air.
3. Evaporation of a liquid from a heat source at the evaporation temperature.
4. Constant pressure heating of a gas by a constant temperature source.
Which of these processes is/are reversible?
An inventor states that his new engine rejects to the sink 40% of heat absorbed from the source while the source and sink temperatures are 327ºC and 27ºC respectively. His engine is therefore equivalent to
An inventor states that his new conceptual engine, while operating between temperature limits of 377oC and 27oC, will reject 50% of heat absorbed from the source. What type of cycle will this engine have?
An engine operates between temperature limits of 900 K and T2 and another between T2 and 400 K. For both to be equally efficient, the value of T2 will be
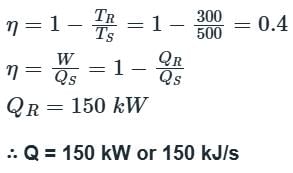
A heat engine is supplied with 250 KJ/s of heat at a constant fixed temperature of 227°C. The heat is rejected at 27°C. The cycle is reversible, if the amount of heat rejected is:
|
29 docs|220 tests
|
|
29 docs|220 tests
|