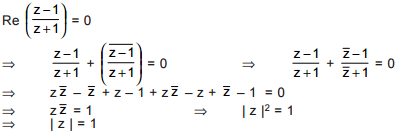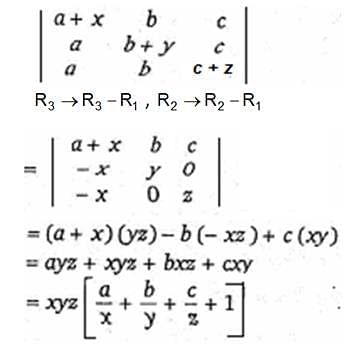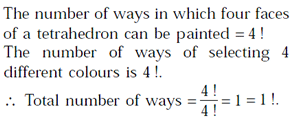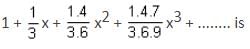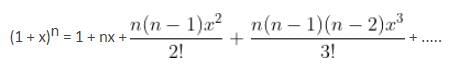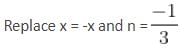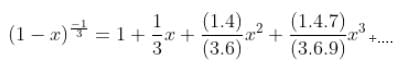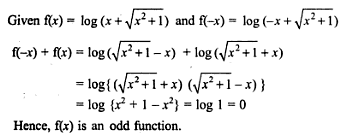KIITEE Mock Test - 1 - JEE MCQ
30 Questions MCQ Test - KIITEE Mock Test - 1
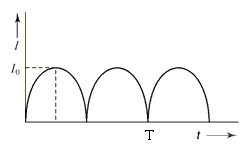
The output current (I) versus time (t) curve of a rectifier is shown in the figure. The average value of the output current in this case is

The angle of minimum deviation for a prism of refractive index 3/2 is equal to the angle of prism. The angle of prism is
A moving coil galvanometer consists of a coil of N turns and area A suspended by a thin phosphor bronze strip in radial magnetic field B. The moment of inertia of the coil about the axis of rotation is l and C is the torsional constant of the phosphor bronze strip. When a current i is passed through the coil, it deflects through an angle θ (in radians). The current sensitivity of the galvanometer is increased if
A wire of length L metres carrying a current I amperes is bent in the form of a circle. The magnitude of the magnetic moment is
Two diodes have a resistance of 20 ohm and are centre-tapped with rms secondary voltage from centre tap to each end of secondary voltage of 50 V. If the external resistance is 980 ohm, then what is the mean load?
If the potential energy of electron in a hydrogen atom is -Ke2/r, then its kinetic energy is
The voltage 7.25 sin (300t) is applied to a series RLC circuit with R = 120 ohms, L = 0.140 H and C = 1.45 μF. What is the impedance Z and the phase angle θ?
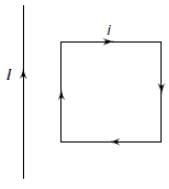
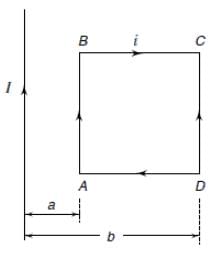
A rectangular loop carrying a current i is situated near a long straight wire such that the wire is parallel to one of the sides of the loop. If a steady current is established in the wire, as shown in the figure, the loop will
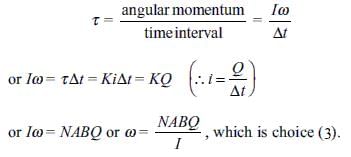
A moving coil galvanometer consists of a coil of N turns and area A, suspended by a thin phosphor bronze strip in radial magnetic field B. The moment of inertia of the coil about the axis of rotation is l and C is the torsional constant of the phosphor bronze strip. When a current i is passed through the coil, it deflects through an angle θ (in radians).
When a charge Q is passed almost instantly through the coil, the angular speed ω acquired by the coil is
By ignoring Aufbau principle, if each orbital accommodates three electrons instead of two, then the incorrect statement(s) about the new electronic arrangement of Zr atom having atomic number 40 is/are:
Solubility product of a salt AB is 1 x 10-8 M2 in a solution in which the concentration of A+ ions is 10-3 M. The salt will precipitate when the concentration of B- ions is kept
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because of
Directions: Consider the IUPAC names.
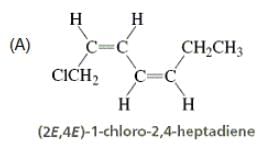
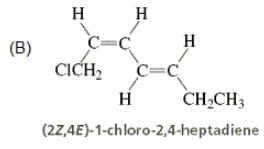
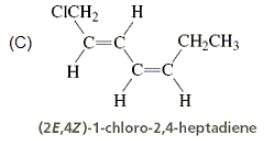
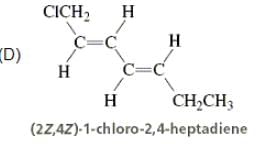
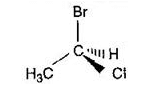
The incorrectly matched pairs are
If 10-4 dm3 of water is introduced into a 1.0 dm3 flask at 300 K, then how many moles of water are in the vapour phase when the equilibrium is established?
(Given: Vapour pressure of H2O at 300 K is 3170 Pa and R = 8.314 J K-1 mol.)
A hypothetical reaction X2 + Y2 → 2XY follows the following mechanism:
X2 → X + X ... fast
X + Y2 → XY + Y … slow
X + Y → XY … fast
The order of the overall reaction is
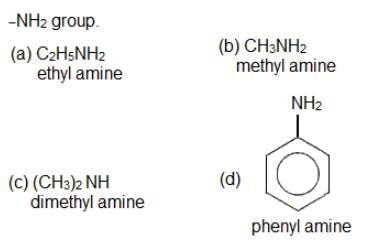
Which of the following amines will not undergo carbylamine reaction?
During acetylation of amines, what is replaced by acetyl group?
A hydrocarbon contains 10.5 g of carbon per g of H. If one litre vapour of hydrocarbon at 127°C and 1 atm pressure weighs 2.8 g, then the molecular formula of hydrocarbon is
The median of a grouped data is 39.8. The lower limit of the median class is 35 and the frequency is 10. The cumulative frequency of the preceding class of the median class is 34 and the total number of observations is 80. The class size of the grouped data is
The interval in which x (> 0) must lie so that the greatest term in the expansion of (1 + x)2n has the greatest coefficient is
Coefficients of variation of two distributions are 50 and 60 and their arithmetic mean are 30 and 25 respectively. Difference of their standard deviation is
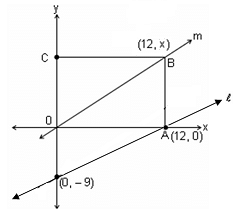
In the given figure, OABC is a rectangle. If || m, what is the area of the rectangle OABC?
The number of ways in which four faces of a regular tetrahedron can be painted with four different colours is


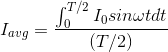
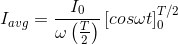
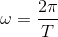 .
.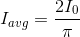
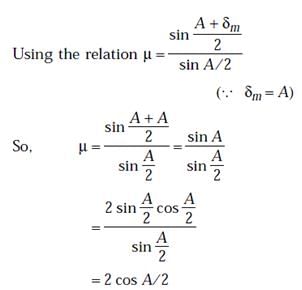


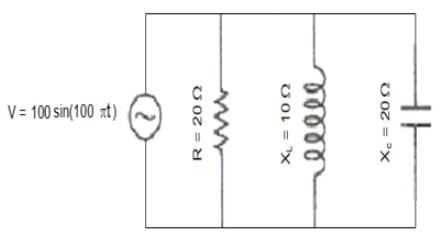
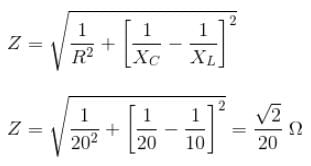
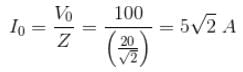
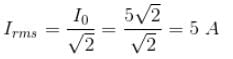
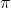 r2I, where r is the radius of the circular loop.
r2I, where r is the radius of the circular loop.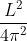
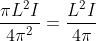
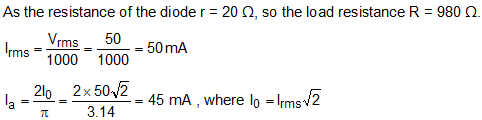
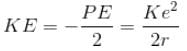
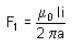
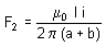
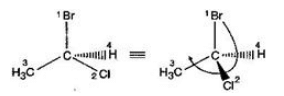
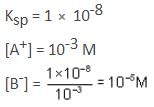
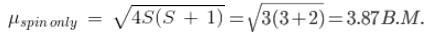
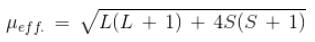
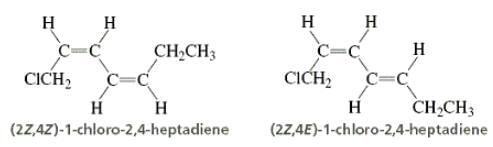
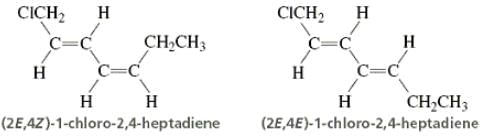
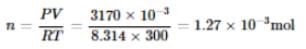
 ... is
... is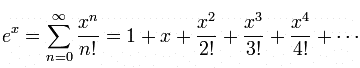
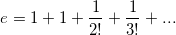
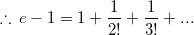
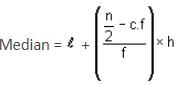
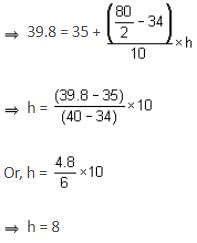
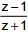 is purely imaginary, then | z | =?
is purely imaginary, then | z | =?