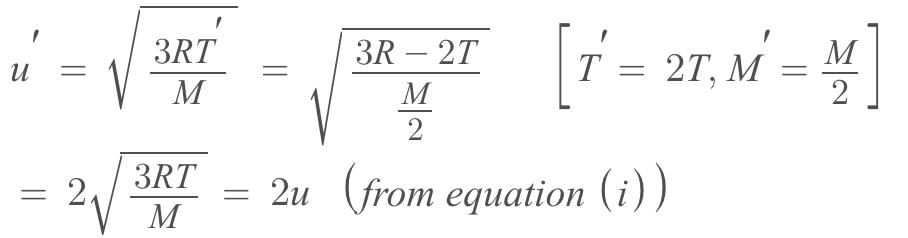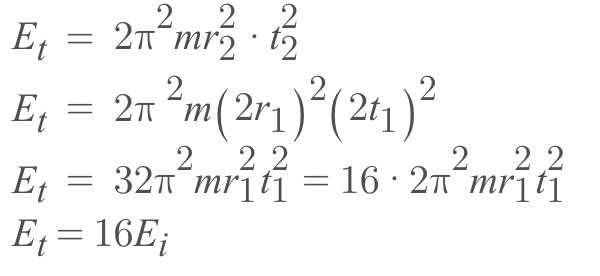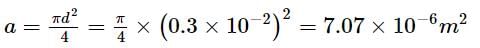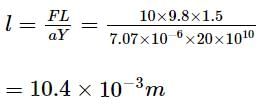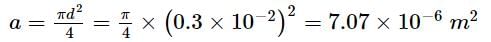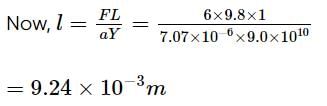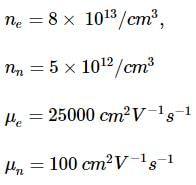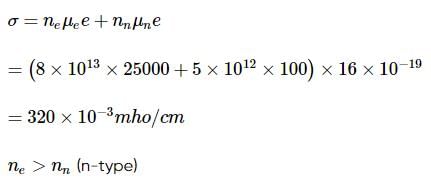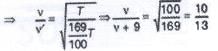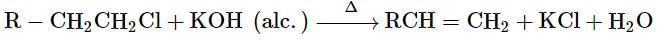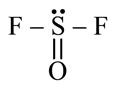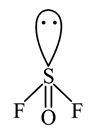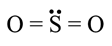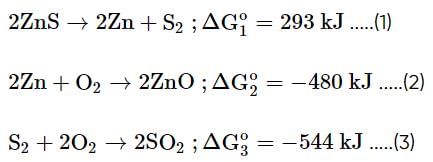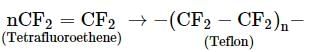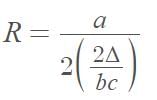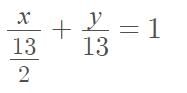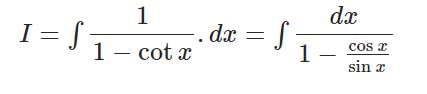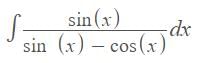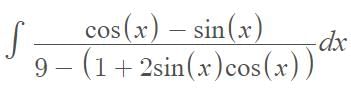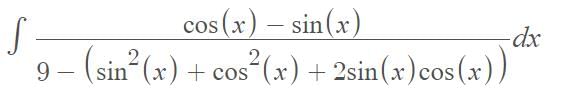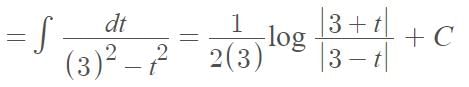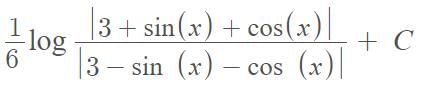KIITEE Mock Test - 10 - JEE MCQ
30 Questions MCQ Test - KIITEE Mock Test - 10
Three point masses each of mass ‘m’ are kept at the corners of an equilateral triangle of side ‘L’. The system rotates about the center of the triangle without any change in the separation of masses during rotation. The period of rotation is directly proportional to (cos 300 = sin600 √3/2)
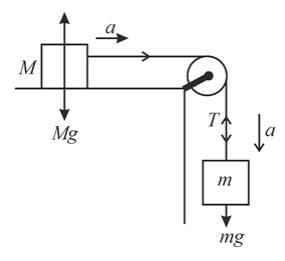
Two blocks of masses M and m are connected by a string passing over a pulley as shown in the figure.
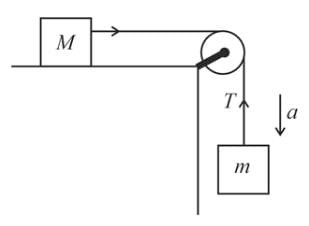
The downward acceleration of the block with mass m is

The downward acceleration of the block with mass m is
The rms speed of oxygen molecule in a gas is u, If the temperature is doubted and the molecules dissociates into two atoms, the rms speed will be
If the radius of the circular path and frequency of revolution of a particle of mass m are doubled, then the change in its kinetic energy will be (Ei and Er are the Initial and final kinetic energies of the particle respectively.)
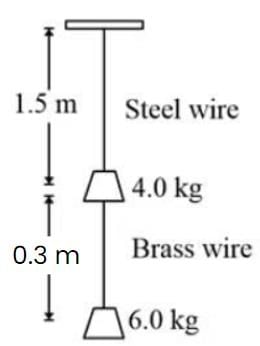
Find the elongation of the steel and brass wire in the fig. unloaded length of steel wire = 1.5 m, unloaded length of brass wire 0.3 cm, diameter of each wire = 0.3 cm. Young's modulus for steel = 20 × 1010 Pa and that for brass = 9.0 × 1010 Pa.
A semiconductor has an electron concentration of 8×1013 per cm3 and a hole concentration of 5 x 1012 per cm3. The electron mobility is 25000 cm2 V−1 s−1
and the hole mobility is 100 cm2 V. s−1 . Then,
The fundamental frequency of the sonometer wire Increases by 9 Hz, if its tension is increased by 69%, keeping the length constant. The frequency of the wire is
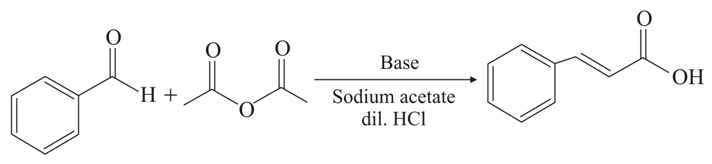
In which reaction, an aromatic aldehyde is treated with acetic anhydride, in presence of the corresponding salt of the acid, to give an unsaturated aromatic acid?
Calculate the value of ΔG for the reaction given below at 800°C:
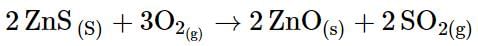
Using the following data given (at same temperature),
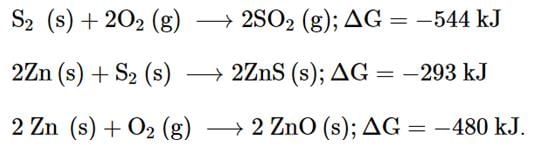
The products obtained when anisole is heated in a sealed tube with HI are
A hypothetical reaction X2 + Y2 → 2XY follows the following mechanism:
X2 → X + X ... fast
X + Y2 → XY + Y … slow
X + Y → XY … fast
The order of the overall reaction is
Which of the following is formed by free radical polymerisation?
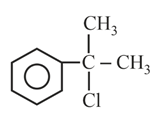
Cannizzaro reaction is not shown by which one of the following compound?
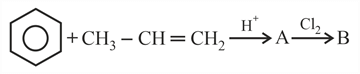
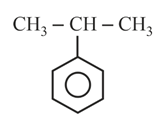
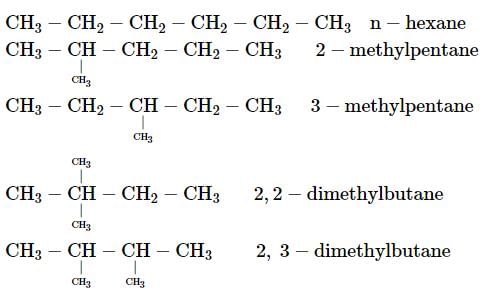
How many chain isomers could be obtained from the alkane C6H14?
The solution of the differential equation dθ/dt = - k(θ - θ0) where k is constant, is …….
If b + c, c + a, a + b are in H.P., then a2, b2, c2 will be in
II A is non-singular matrix and (A + l) (A - l) = 0 then A + A-1 = ....
The y-intercept of the line passing through A(6, 1) and perpendicular to the line x - 2y = 4 is ........
The fourth, seventh, and tenth terms of a G.P. are p, q, and r, respectively. Which of the following is true?
a and b are non-collinear vectors. If c = (x - 2) a + b and d = (2x + 1)a - b are collinear vectors, then the value of x = .......
Which of the following can not be the direction cosines of a line?



 as λ decreases on entering in water, β also decreases.
as λ decreases on entering in water, β also decreases.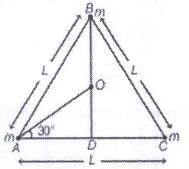
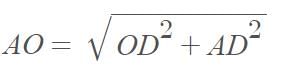
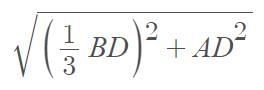
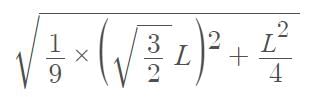
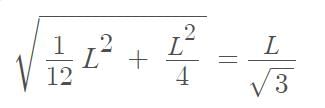
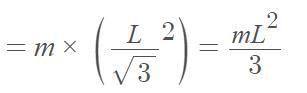 …(i)
…(i)
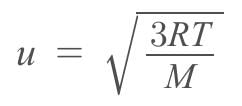 …(i)
…(i)