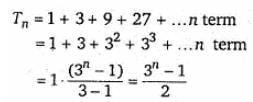KIITEE Mock Test - 8 - JEE MCQ
30 Questions MCQ Test - KIITEE Mock Test - 8
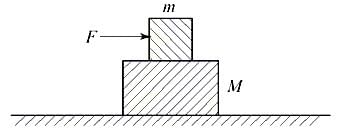
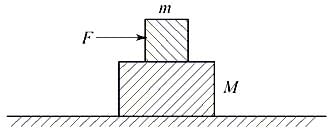
A block of mass m is lying on another block of mass M, lying on a horizontal frictionless surface as shown in the figure. The coefficient of static friction between the two blocks is . If a force F (> mg) is applied to the block of mass m, the acceleration with which the block of mass M will move on the horizontal surface is given by (Here, is the coefficient of kinetic friction between the two blocks)

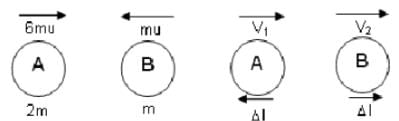
Spheres A and B of equal radius and of masses 2 m and m, respectively, are moving towards each other and strike directly. The speeds of A and B before the collision are 3u and u, respectively. The collision is such that B experiences an impulse of 4mcu, where c is constant.
What is the coefficient of restitution?
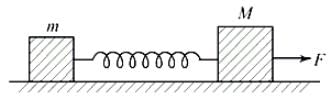
Two blocks of masses m and M are placed on a horizontal frictionless table connected by a spring as shown in the figure. The block of mass M is pulled to the right with a force F. If the acceleration of the block of mass m is 'a', then the acceleration of the block of mass M will be

A smooth sphere 'A' is moving on a frictionless horizontal surface with angular speed ω and centre of mass velocity v. It collides elastically and head-on with an identical sphere B at rest. Neglect friction everywhere. After the collision, their angular speeds are ωA and ωB, respectively. Then,
A solid cylinder of mass M and radius R rolls down an inclined plane of height 'h'. The angular velocity of the cylinder when it reaches the bottom of the plane will be
A force 'F' is applied on a square plate of side 'L'. If percentage error in determination of 'L' is 3% and 'F' is 4%, then the permissible error in pressure is
Which of the following statements is true in context of the solutions of alkali metals?
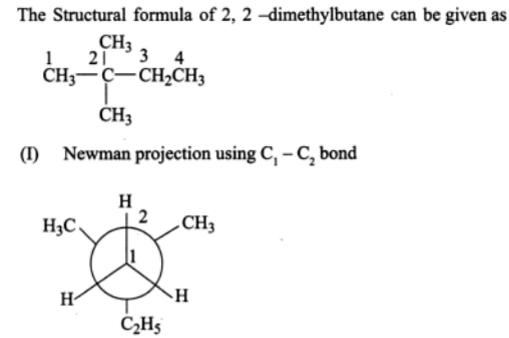
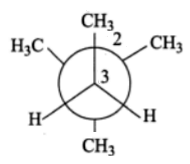
In the Newman projection for 2, 2-dimethylbutane, X and Y, respectively, will be
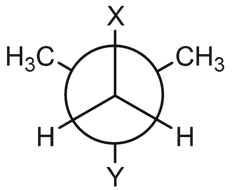
A. H and H
B. H and C2H5
C. C2H5 and H
D. CH3 and CH3
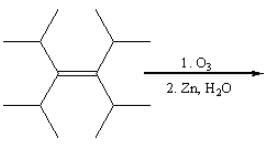
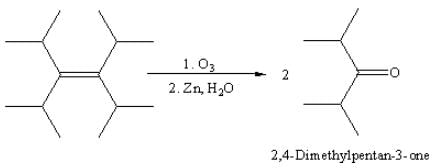
Acid hydrolysis of which of the following compounds yields two different organic compounds?
t1/4 can be taken as the time taken for the concentration of a reactant to drop to 3/4 of its initial value. If the rate constant for a first order reaction is k, then t1/4 can be written as
The correct statement about the compounds (A), (B) and (C) is:
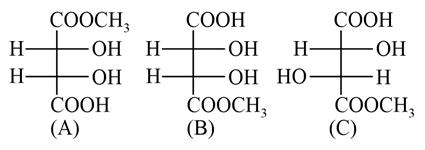
Find the flocculating value of the effective ion formed when 4 ml of 0.005 M solution of barium chloride is added to 16 ml solution of arsenic sulphide after complete coagulation of 2 hours.
The following equilibria are given:
a. N2 + 3H2 2NH3; K1
b. N2 + O2 2NO; K2
c. H2 + O2
H2O; K3
The equilibrium constant for the reaction,
2NH3 + O2
2NO + 3H2O in terms of K1, K2 and K3 will be
Which of the following statements is/are incorrect?
A. The complex ion trans-dichloro bis (ethylenediamine) rhodium(III) is optically active.
B. Secondary valancies are non-directional, whereas primary valencies are directional.
C. Both geometrical and optical types of isomerism are shown by [Co(en)2Cl2]+.
For the reaction 2H2 + O2 2H2O, H = -571 kJ, bond energy of H - H = 435 kJ/mol and that of O - O = 498 kJ/mol. Calculate the average bond energy (in kJ/mol) of O - H bond using this data.
Consider the following reaction:
CaCO3(s) ⇌ CaO(s) + CO2(g)
The decomposition of limestone is non-spontaneous at 298 K.
The ΔHº and ΔSºvalues for the reaction are 176.0 kJ and 160 JK-1, respectively.
At what temperature will the decomposition become spontaneous?
When HCHO is treated with C6H5CHO in the presence of KOH, the products formed are
A solid AB has a NaCl structure. If the radius of cation A+ is 120 pm, then the maximum possible value of the radius of the anion B- will be
Find the area (in square units) of the parallelogram whose sides are x + 2y + 3 = 0, 3x + 4y - 5 = 0, 2x + 4y + 5 = 0 and 3x + 4y - 10 = 0.
An object is moving in clockwise direction around the unit circle x2 + y2 = 1. As it passes through the point (1/2, √3/2), its y-coordinate is decreasing at the rate of 3 units per second. The rate at which the x-coordinate changes at this point is (in units per second)
The probability that a teacher will give an unannounced test during any class meeting is 1/5. If a student is absent twice, then the probability that the student will miss at least one test is
The equation of the family of lines perpendicular to the line 3x + 4y + 7 = 0, is
The sum of n terms of the series 1 + (1 + 3) + (1 + 3 + 9) + (1 + 3 + 9 + 27) + ...... is


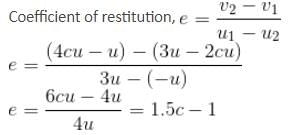


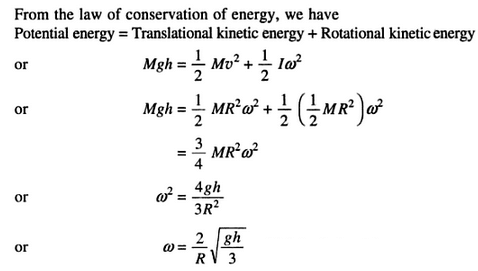
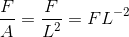
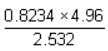 up to correct significant figures is equal to
up to correct significant figures is equal to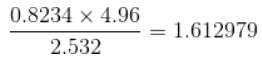
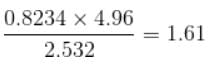
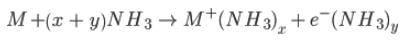

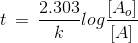
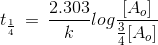
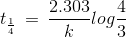
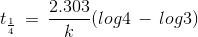
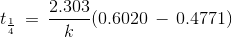
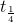 =
= 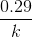
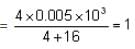
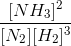
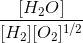
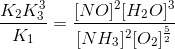

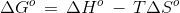
 is a negative value.
is a negative value.

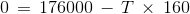
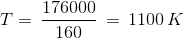
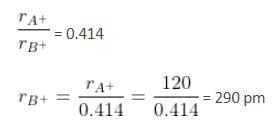
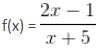 , Where x ≠ -5, then f-1(x) is equal to
, Where x ≠ -5, then f-1(x) is equal to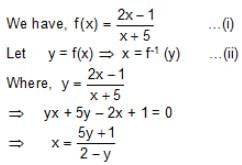
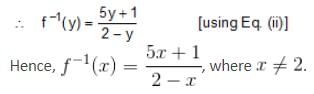
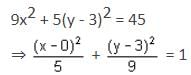
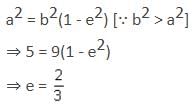
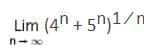 is equal to
is equal to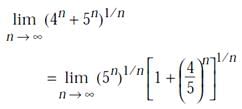
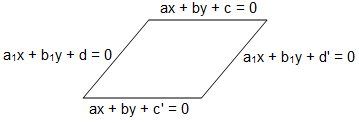
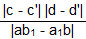
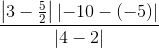 sq. units
sq. units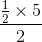 sq. units = 5/4 sq. units
sq. units = 5/4 sq. units