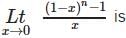MHT CET Mock Test - 1 - JEE MCQ
30 Questions MCQ Test - MHT CET Mock Test - 1
Basic materials used in the present solid state electronic devices like diode, transistor, ICs, etc are
electromagnetic induction i.e currents can be induced in coils (Select the best)
When a satellite moves in a circular orbit, the _______acceleration is provided by the gravitational attraction of the earth
Shown in figure is a Post Office box. In order to calculate the value of external resistance, it should be connected between
When the distance between two charged particle is halved, the force between them becomes
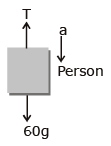
One end of massless rope, which passes over a massless and frictionless pulley P is tied to a hook C while the other end is free. maximum tension that the rope can bear is 360 N. Which what value of maximum safe acceleration (in ms-2) can a man of 60 kg climb down the rope ?
[AIEEE 2002]
An element has configuration 4d55s2. The element belongs to
The hydroxide of alkaline earth metal, which has the lowest value of solubility product (Ksp) at normal temperature (25°C) is-
The chemical reaction in which reactants require high amount of activation energy are generally
Among the following series of transition metal ions, the one where all metal ions have 3d2 electronic configuration is (Atomic number, Ti = 22, V = 23, Cr = 24, Mn = 25)
[AIEEE 2004]
A circle is the set of …… in a plane that are equidistant from a fixed point in the plane.
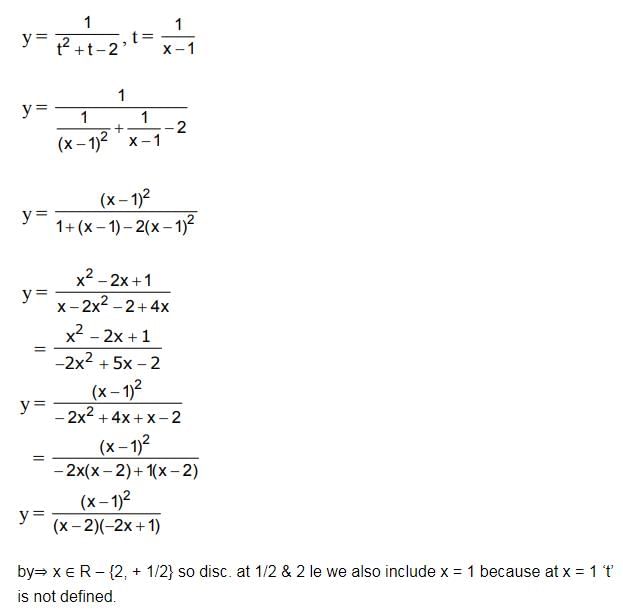
A letter is known to have come either from TATANAGAR or from CALCUTTA. On the envelope just two consecutive letters TA are visible. What is the probability that the letters came from TATANAGAR ?
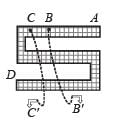
If y = 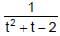 where t =
where t = 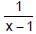 , then the number of points of discontinuities of y = f(x), x ∈ R is
, then the number of points of discontinuities of y = f(x), x ∈ R is



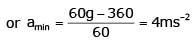
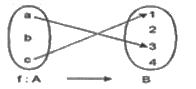
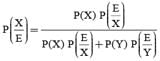
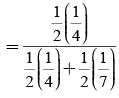
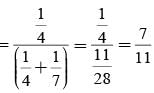
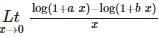 is equal to
is equal to