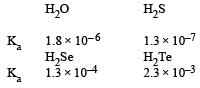31 Year NEET Previous Year Questions: The p-Block Elements - 1 - NEET MCQ
20 Questions MCQ Test - 31 Year NEET Previous Year Questions: The p-Block Elements - 1
Which one of the following orders is not in accordance with the property stated against is ? [2006]
Which one of the following orders correctly represents the increasing acid strengths of the given acids? [2007]
Which one of the following arrangements does not give the correct picture of the trends indicated against it ? [2008]
In the case of alkali metals, the covalent character decreases in the order: [2009]
Among the following which is the strongest oxidising agent? [2009]
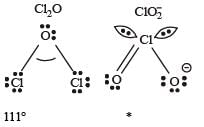
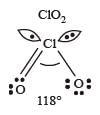
The correct order of increasing bond angles in the following species are : [2010]
Oxidation states of P in H4 P2O5 , H4 P2O6 , and H4 P2O7 , are respectively: [2010]
Which one of the following compounds is a peroxide ? [2010]
Match List - I (substan ces) with List - II (processes) employed in the manufacture of the substances and select the correct option. [2010]

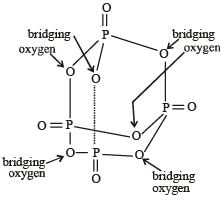
How many bridging oxygen atoms are present in P4O10? [2010]
Which of the following structures is the most preferred and hence of lowest energy for SO3 ? [2011 M]
Which of the following oxide is amphoteric ?
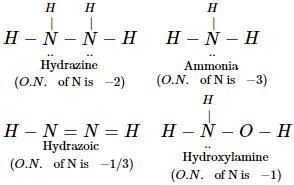
In which of the following compounds, nitrogen exhibits highest oxidation state ? [2012]
Which of the following statements is not valid for oxoacids of phosphorus? [2012]
Sulphur trioxide can be obtained by which of the following reaction : [2012]
Which is the strongest acid in the following : [NEET 2013]
Which of the following does not give oxygen on heating? [NEET 2013]
Which of the following is a polar molecule ? [NEET 2013]
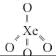
Identify the incorrect statement, regarding the molecule XeO4: [NEET Kar. 2013]
In which of the following arrangements the given sequence is not strictly according to the property indicated against it ? [2012 M]


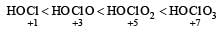

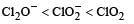
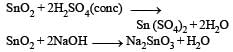
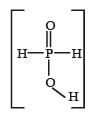 Hypophosphorous acid
Hypophosphorous acid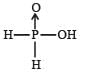
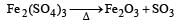
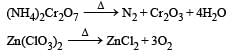
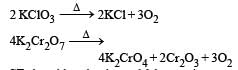
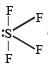 and resultant
and resultant