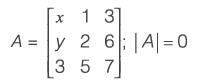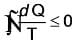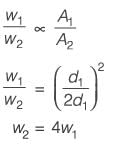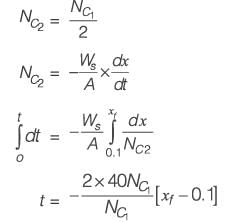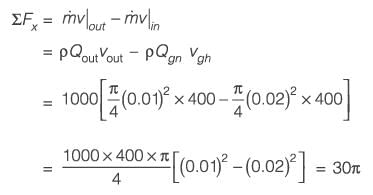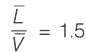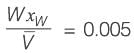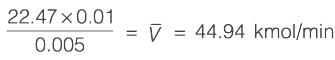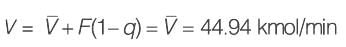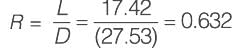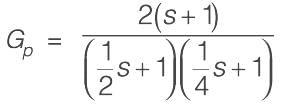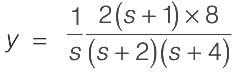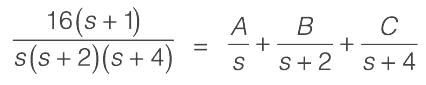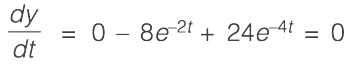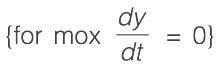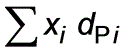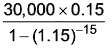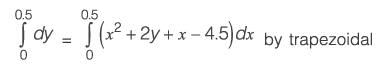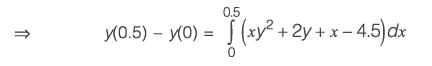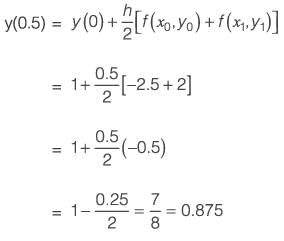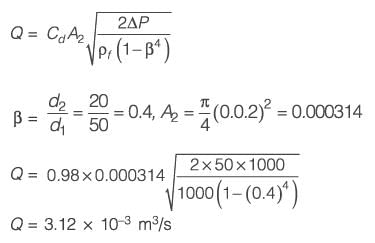GATE Chemical Engineering Mock Test - 1 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 1
In a classroom at NIT Jaipur, the proportion of boys to girls is 3 to 4. If the total number of students is 84, how many boys and girls are there?
Choose the most suitable synonym to fill in the blank:
"After the long hike, we felt completely ________ and needed some time to recuperate."
"After the long hike, we felt completely ________ and needed some time to recuperate."
Inhaling smoke from a burning _________ could cause _________ to you rapidly.
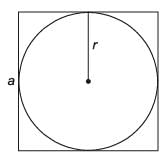
A sphere with a radius of r cm is placed inside a cube-shaped box.
What is the minimum volume (in cm3) of the box required to contain the sphere?
Rice is an essential and affordable carbohydrate source that plays a vital role in diets around the globe. The impact of climate change, leading to severe weather conditions, threatens the consistent availability of rice. Researchers are focused on creating Green Super Rice (GSR), which can endure extreme weather while producing sustainable higher yields.
Which of the following represents the CORRECT logical conclusion drawn from the information presented in the passage above?
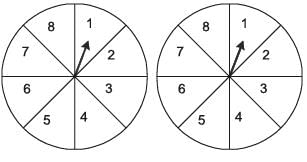
A game involves spinning an arrow around a fixed disk, as illustrated below. When the arrow stops, it can land on one of eight equally likely sectors, numbered 1 through 8 as depicted. In this game, two such disks are utilized, with their arrows spun independently.
What is the probability that the sum of the numbers on the sectors where the arrows land after spinning the two disks equals 8?
Examine the following inequalities.
(i) 3p - q < 4
(ii) 3q - p < 12
Which of the following expressions listed below fulfills the conditions set by the two inequalities?
A task can be accomplished by 12 workers in 15 days, with each working 8 hours daily. After 5 days of work, 4 workers exit the project. In order to complete the task within the original timeframe, the remaining workers decide to increase their daily working hours. How many hours must the remaining workers work each day to finish the task on schedule?
12 hours per day
Which device is responsible for transforming a mechanical displacement into an electrical signal?
In a P&ID diagram, which symbol is commonly used to denote a valve?
For the matrix  , determine the ordered pair (x, y) such that det(A) = 0.
, determine the ordered pair (x, y) such that det(A) = 0.
What is the value of the cyclic integral of dQ/T for a process that is irreversible?
A solid metallic object is immersed in water. Its weight in air is 100 N, while its weight when completely submerged in water is 80 N. Calculate the buoyant force acting on the object.
What is the total energy/heat needed to completely vaporize 10 g of water starting from 0º C? (Given that the heat capacity of water is 4.2 J/g.K and the ΔH vaporization of water is 2260 kJ/kg).
Consider two stationary spherical droplets of pure water with diameters d1 and 2d1. CO2 diffuses into these droplets from the surrounding environment. If the diffusion rate of CO2 into the smaller droplet is W1 mol s-1, what is the rate of CO2 diffusion into the larger droplet?
In the production of soap, the triglycerides found in fats and oils undergo hydrolysis primarily to yield
A moist solid with 20% (w/w) moisture (calculated based on the mass of the bone-dry solid) is subjected to drying in a tray-dryer. The solid has a critical moisture content of 10% (w/w). For the initial 4 hours, the drying rate (kg m-2 s-1) remains constant, after which it reduces linearly to half its original rate over the next hour. At the conclusion of 5 hours of drying, the moisture content percentage of the solid is ________ % (w/w) (rounded to one decimal place).
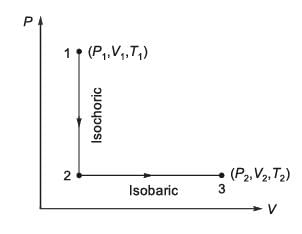
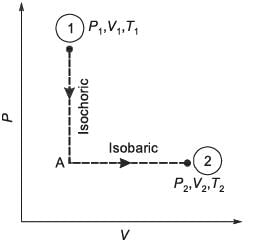
A total of N moles of an ideal gas undergo a two-step transformation as illustrated in the figure. Let P, V, and T represent the pressure, volume, and temperature of the gas, respectively. Starting from state-1 (P1, V1, T1), the gas first undergoes an isochoric process (constant volume) to reach state-A, followed by an isobaric expansion (constant pressure) to arrive at state-2 (P2, V2, T2). For an ideal gas, the relationship CP - CV = NR holds true, where CP and CV denote the heat capacities at constant pressure and constant volume, respectively, and are presumed to be independent of temperature. The total heat absorbed by the gas during this two-step process is represented by
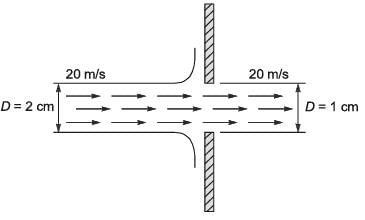
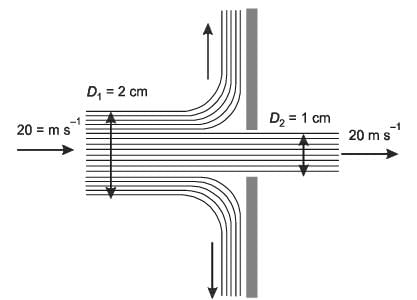
A horizontal cylindrical jet of water with a diameter D1 = 2 cm impacts a vertical solid plate that has a hole of diameter D2 = 1 cm, as illustrated in the figure. Part of the jet flows through the hole while the remainder is redirected along the surface of the plate. The density of water is 1000 kg m-3. Given that the velocity of the jet is 20 m s-1, calculate the magnitude of the horizontal force, in N, necessary to keep the plate stationary.
In a heat exchanger, the log mean temperature difference (LMTD) for a counter-flow arrangement is invariably:
In a reaction that follows Michaelis-Menten kinetics, what does the reaction rate approach when the substrate concentrations are significantly high?
An evaporator with a heat transfer area of 70 m2 is used to concentrate a salt solution by utilizing steam. The feed rate and temperature of the salt solution are 10000 kg h-1 and 40°C, respectively. The feed rate and temperature of the saturated steam are 7500 kg h-1 and 150°C, respectively. In the evaporator, the boiling temperature of the solution is 80°C. The average specific heat of the solution is 0.8 kcal kg-1 K-1. The latent heat of vaporization is 500 kcal kg-1. If the steam economy is 0.8, what is the overall heat transfer coefficient in kcal h-1 m-1 K-1 (rounded to the nearest integer)?
A binary mixture with equal molar amounts is to be separated using a simple tray-distillation column. The feed flow rate is 50 kmol min-1. The mole fractions of the more volatile component in the distillate and bottoms are 0.90 and 0.01, respectively. Both the feed and the reflux stream are saturated liquids. Using the McCabe-Thiele method, the operating line for the stripping section is defined as
y = 1.5 x - 0.005
where y and x represent the mole fractions of the more volatile component in the vapor and liquid phases, respectively. The reflux ratio is ______ (rounded to two decimal places).
Consider a system with a single input and single output (SISO) defined by the transfer function
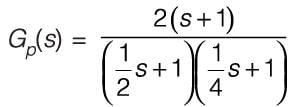
where the time constants are measured in minutes. The system receives a unit step input commencing at time t = 0. Calculate the time at which the output response reaches its peak value, rounded to _________ minutes (to two decimal places).
For a cube with side ‘a’ the sphericity comes out to be 'ϕ1' if the side of cube is changed to ‘2a’ and sphericity becomes 'ϕ2'. what will be the ratio of
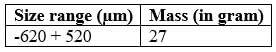
Using the provided particle size analysis, determine the mass mean diameter. Please present your answer rounded to two decimal places in (μm).
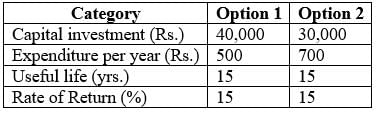
What is the overall yearly expense for option 2?
(in rupees rounded to the nearest whole number) …………………………….
In a distillation column operating under conditions of fully mixed vapor and liquid streams, what is the relationship between the point efficiency and the Murphree efficiency in terms of their ratio? (Provide your answer as an integer) ………………
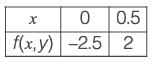
The differential equation dy/dx = xy2 + 2y x - 4.5, along with the initial condition y(0) = 1, is to be solved using a predictor-corrector method. Employ a predictor based on the implicit Euler's method and a corrector that utilizes the trapezoidal rule of integration, both with a step size of 0.5. Focusing solely on positive values of y, determine the value of y at x = 0.5, rounded to three decimal places.
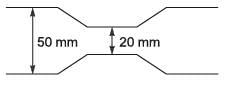
A venturi meter with a venturi coefficient, Cv = 0.98, is installed in a pipe that has an inner diameter of 50 mm. Water, with a density of 1000 kg m-3, is flowing through this pipe. The pressure drop recorded across the venturi meter is 50 kPa. Given that the diameter of the venturi throat is 20 mm, what is the estimated flow rate of water expressed in ______ x 10-3 m3 s-1 (rounded to two decimal places)?