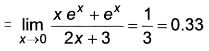GATE Chemical Engineering Mock Test - 2 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 2
What is a significant issue that Donald Trump has emphasized in his 2024 presidential campaign?
In an adiabatic plug flow reactor, the energy balance equation is utilized to determine the conversion X. Two conclusions are drawn as follows:
Statement 1: Conversion can be expressed as the heat required to elevate the feed stream to temperature T’ compared to the heat released from the reaction at the initial temperature Ti.
Statement 2: Conversion can be described as the ratio of the heat necessary to increase the feed stream temperature to T’ against the heat released by the reaction at T’.
Select the correct option:
Statement 1: Conversion can be expressed as the heat required to elevate the feed stream to temperature T’ compared to the heat released from the reaction at the initial temperature Ti.
Statement 2: Conversion can be described as the ratio of the heat necessary to increase the feed stream temperature to T’ against the heat released by the reaction at T’.
Select the correct option:
What is the name of the chemical reaction shown in the image?

In the petroleum sector, catalytic reforming is frequently utilized to enhance the quality of fuels. The reaction that is considered undesirable during the catalytic reforming of naphtha is
A cube made of metal has a side length of 10 cm at a temperature of 20°C. Given that the coefficient of volume expansion for the metal is β = 5 x 10-5 per°C, what will be the change in the cube's volume when the temperature rises to 30°C?
A distillation column is designed to separate an ideal binary mixture to achieve specific purities for the distillate and bottoms at a designated column pressure. Given that RRmin represents the minimum reflux ratio necessary for this separation, identify the statement that is NOT CORRECT regarding how the total annualized cost (TAC) of the column varies with the reflux ratio (RR).
A body receives a total incident radiant energy of 2200 W/m2, which it partially reflects, absorbs, and transmits. Among this energy, 300 W/m2 is reflected and 800 W/m2 is absorbed by the body. What will be the transmissivity of the body?
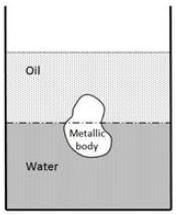
The large container depicted in the image holds oil and water. An object is immersed at the boundary between the oil and water, with 45 percent of its volume in oil and the remaining portion in water. What is the density of the object in _________ kg/m3? The specific gravity of oil is 0.7, and the density of water is 1000 kg/m3. The acceleration due to gravity is g = 10 m/s2.
A second-order system characterized by the transfer function, 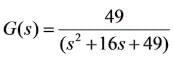 starts from rest and is subjected to a step input. What will be the peak percentage overshoot observed in the system's response?
starts from rest and is subjected to a step input. What will be the peak percentage overshoot observed in the system's response?
The unit associated with the following property is m2/s-1.
Two extensive parallel flat walls are kept at temperatures of 1000 K and 500 K, respectively. Radiation shields are to be placed between these two walls. It is assumed that the emissivities of both the walls and the shields are the same. Given that the melting temperature of the shields is 900 K, what is the maximum number of shield(s) that can be positioned between the walls?
Saturated steam condenses on a vertical plate that is kept at a constant temperature. Let x represent the vertical distance from the top edge of the plate. The local heat transfer coefficient h(x) is proportional to Γ(x)-1/3, where Γ(x) denotes the local mass flow rate of the condensate per unit width of the plate. What is the ratio of the average heat transfer coefficient across the entire plate to the heat transfer coefficient at the bottom of the plate?
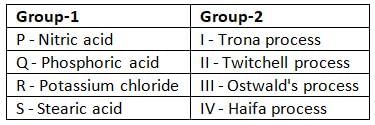
Associate the items listed in Group-1 with their corresponding manufacturing processes in Group-2. Identify the correct pairings from the combinations provided in the image below.
In a shell and tube heat exchanger, how can the heat transfer area be enhanced?
A numerical solution for the equation f(x) = x √(x - 3) = 0 can be found using the Newton-Raphson method. If the initial value for the iteration is x = 2, what is the value of x to be used in the subsequent step?
Which of the following statements are CORRECT according to the Surface Renewal Theory of mass transfer?
In a catalytic process happening within a packed bed reactor, which of the following statements are accurate?
A material at a temperature of 4°C possesses a thermal expansion coefficient 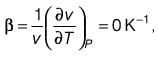 and an isothermal compressibility
and an isothermal compressibility 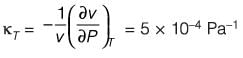 along with a molar volume of v = 18 × 10-6 m3 mol-1. If s denotes the molar entropy, then at 4°C, the calculated value of
along with a molar volume of v = 18 × 10-6 m3 mol-1. If s denotes the molar entropy, then at 4°C, the calculated value of 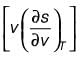 for the substance is ______ J mol-1 K-1 (rounded to the nearest integer).
for the substance is ______ J mol-1 K-1 (rounded to the nearest integer).
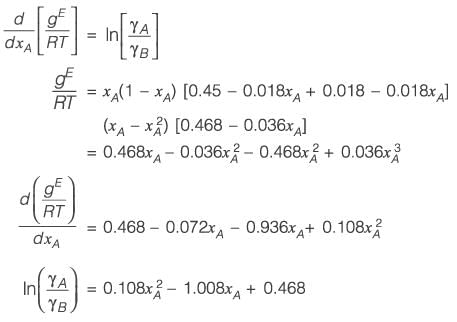
The molar excess Gibbs free energy (gE) of a liquid mixture comprising components A and B is expressed as
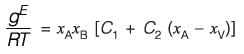
where xA and xB represent the mole fractions of A and B, respectively, R is the universal gas constant (8.314 J K-1 mol-1), T denotes the temperature in Kelvin, and C1 and C2 are parameters that vary with temperature. At a temperature of 300 K, the values are C1 = 0.45 and C2 = -0.018. If γA and γB indicate the activity coefficients for A and B, respectively, calculate the value of
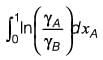
at 300 K and 1 bar, rounding off to the nearest integer.
In a cake filtration process operating at a constant rate, the volumes of filtrate collected are 120 m3 at 1 minute and 240 m3 at 2 minutes. Considering the cake resistance remains constant and the resistance of the filter medium is negligible, if the pressure drop across the cake is 10 kPa at 1 minute, what is its value at 2 minutes________kPa (rounded to the nearest integer)?
A cylindrical fin with a diameter of 24 mm is mounted horizontally on a vertical flat wall. The heat transfer rate from the fin to the ambient air is 60% of what it would be if the entire fin were at the wall temperature. Given that the fin effectiveness is 10, determine the length of the fin in mm, rounding to the nearest integer.
An elementary irreversible reaction in the gas phase, represented as A → B C, is performed at constant temperature and pressure in two different types of ideal reactors:
(i) a 10 m3 plug flow reactor (PFR),
(ii) a 10 m3 continuous-stirred tank reactor (CSTR).
When pure A is introduced at a rate of 5 m3 h-1 into the PFR operating at 400 K, the conversion achieved is 80%. Conversely, if a mixture containing 50 mol% of A and 50 mol% of an inert substance is supplied at 5 m3 h-1 to the CSTR functioning at 425 K, the conversion remains 80%. The universal gas constant R is 8.314 J mol-1 K-1. Assuming an Arrhenius rate law, the estimated activation energy is _________ kJ mol-1 (rounded to one decimal place).
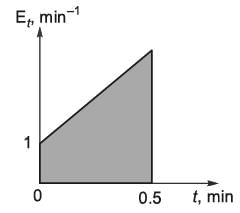
An irreversible liquid-phase reaction of elementary nature, 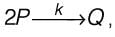 occurs in a non-ideal reactor at constant temperature. The rate constant is given as k = 2 L mol-1 min-1. The E-curve from a tracer study is illustrated in the figure. A concentration of pure P (2 mol L-1) is introduced into the reactor. By applying the segregated model, the percentage conversion of P at the reactor's outlet is _________ % (rounded to the nearest integer).
occurs in a non-ideal reactor at constant temperature. The rate constant is given as k = 2 L mol-1 min-1. The E-curve from a tracer study is illustrated in the figure. A concentration of pure P (2 mol L-1) is introduced into the reactor. By applying the segregated model, the percentage conversion of P at the reactor's outlet is _________ % (rounded to the nearest integer).
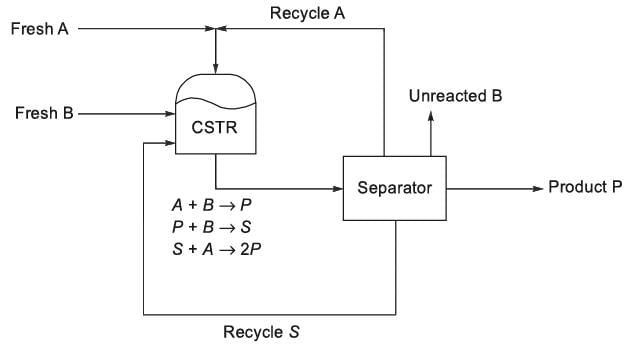
Consider the process illustrated in the figure. The elementary reactions in the liquid phase occur within a continuous stirred tank reactor (CSTR), where Xj represents the mole fraction of the / h component (j = A, B, P, S) in the CSTR. It is noted that k2 = k3. All streams related to the process feed, exit, and recycle are pure. At steady state, the net generation rate of the undesired product, S, within the CSTR is zero. As q = xA/xB is modified at a constant reactor temperature, the reactor volume is adjusted to keep a consistent single-pass conversion of B. For a specified product rate and a 90% conversion of B in the reactor, what is the value of q that minimizes the total molar flow rates of the A and S recycle streams? Please round off your answer to one decimal place.
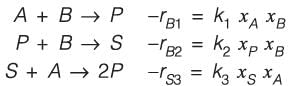
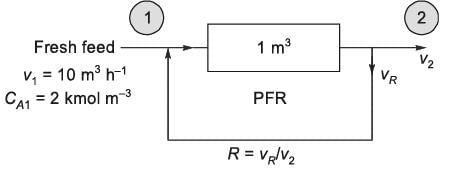
An irreversible liquid-phase reaction of the type 2A → B is conducted at constant temperature in a 1 m3 ideal plug flow reactor (PFR) as illustrated in the figure. The volumetric flow rate of the incoming fresh A is v1 = 10 m3 h-1, with a concentration of CA1 = 2 kmol m-3. With a recycle ratio R = 0, the conversion of A at position 2, relative to the fresh feed at position 1, is 50%. For R → ∞, what is the corresponding conversion of A, expressed as ________ % (rounded to one decimal place)?
The vapor pressures of components A and B are specified as 4 atm and 1.5 atm, respectively.
A liquid feed containing 0.32 moles of component A undergoes vaporization. Determine the mole fraction of component A in the vapor phase.
Given: The products of components A and B are in equilibrium.
Determine the duration required to drain a cone filled with syrup that will be used to coat the cake.
Data :
Height of syrup = 25 cm at t = 0
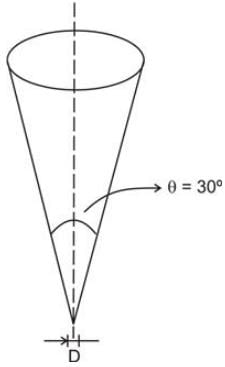
Bottom diameter = 0.5 cm
The flow is considered to be viscous, streamlined, and incompressible.
(Provide your answer in seconds)
What is the value of 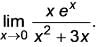
(Upto three decimal places)
An irreversible first-order reaction A → B takes place in a batch reactor. The starting concentration of A is 2 mol/L, and the rate constant k is 0.1 min−1.
What will be the concentration of A after 15 minutes, _______ in mol/L, rounded to two decimal places?



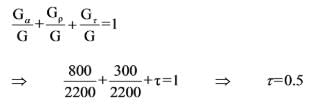
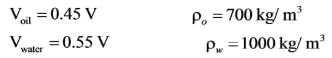
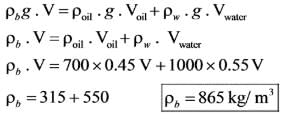
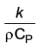
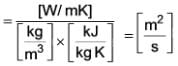
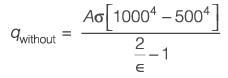 ....(i)
....(i)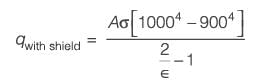

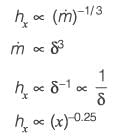
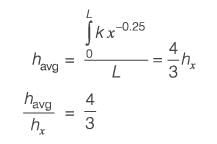
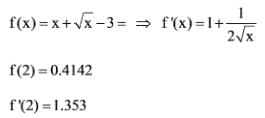
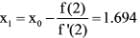
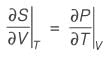
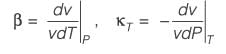

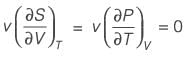
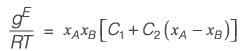

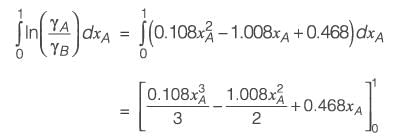
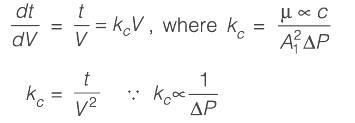
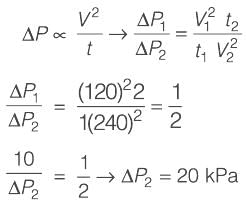
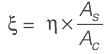
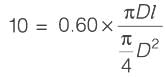

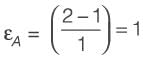
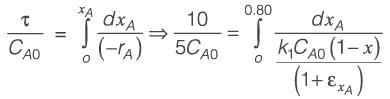
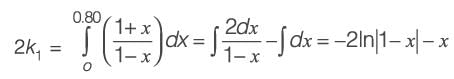
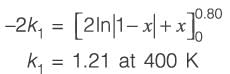
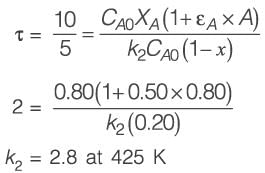

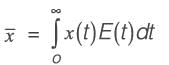
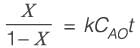
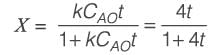
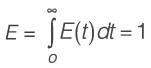 [Area will be one]
[Area will be one]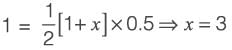
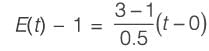
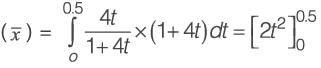
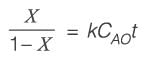
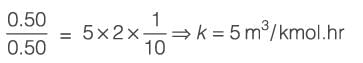
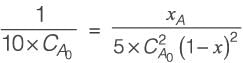
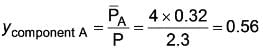
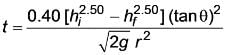 ; 32.44 seconds
; 32.44 seconds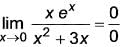 (Apply L’ Hospital Rule)
(Apply L’ Hospital Rule)