GATE Chemical Engineering Mock Test - 4 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 4
During the timeframe from 12:05:00 hours to 12:55:00 hours, how many instances will the second-hand and minute-hand of a clock intersect each other?
What does the idiom "to have an axe to grind" signify?
The following chart illustrates the Installed Capacity (MW) of four different power generation technologies, T1, T2, T3, and T4, along with their Electricity Generation (MWh) over a period of 1000 hours (h).
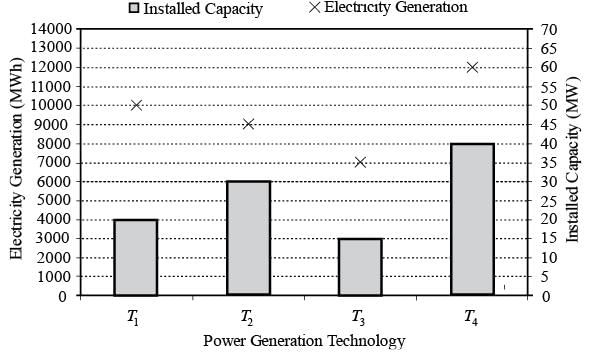
The Capacity Factor for a power generation technology is defined as:

Which of the technologies listed has the greatest Capacity Factor?
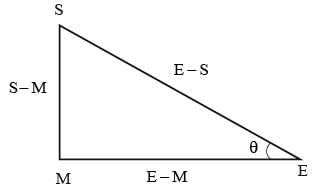
In the half-moon phase, the Earth, Moon, and Sun create a right triangle. If the angle formed by the Moon-Earth-Sun at this half-moon phase is recorded as 89.850 degrees, what is the closest ratio of the distances between Earth-Sun and Earth-Moon?
In a contest featuring two candidates, A and B, 30% of the electorate chose not to cast their votes, and there were 1600 invalid votes. The candidate A emerged victorious, obtaining 4800 more votes than candidate B. A received 51% of the total votes listed on the voter roll. What proportion of the votes did the defeated candidate, B, acquire from the overall number of voters on the list?
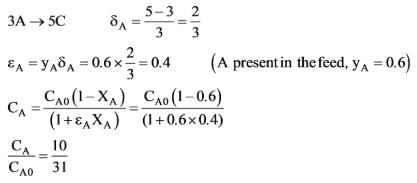
A feed stream containing 60 mole% A and 40 mole% inerts is introduced into a plug flow reactor, where the conversion of A at the reactor's outlet is 60%. What is the ratio CA/CAO at the outlet of the reactor if the reaction kinetics are described by the equation 3A → 5A?
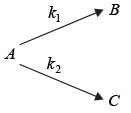
In a well-mixed isothermal batch reactor, there are two parallel first-order liquid phase reactions 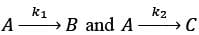 . The reactor starts with an initial concentration of A at 1 kmolm-3, while the concentrations of B and C are initially zero. After a duration of 2 hours, the concentration of A decreases to half of its original value, and the concentration of B is observed to be twice that of C. What are the rate constants k1 and k2, expressed in h-1?
. The reactor starts with an initial concentration of A at 1 kmolm-3, while the concentrations of B and C are initially zero. After a duration of 2 hours, the concentration of A decreases to half of its original value, and the concentration of B is observed to be twice that of C. What are the rate constants k1 and k2, expressed in h-1?
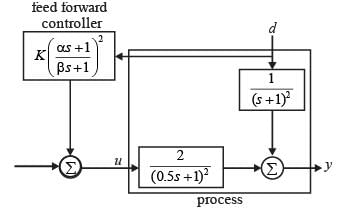
Examine the block diagram depicted in the image, which includes the control input u, disturbance d, and output y. For the feedforward controller, the corresponding ordered pair (K, α/β) is
Which of the following statements is accurate concerning gas-liquid contactors used for mass transfer purposes?
In a facility for ammonia production, hydrogen is produced from methane. The facility comprises the following process units – P: Methanator, Q: CO shift converter, R: CO2 stripper, S: Reformer, T: Ammonia converter. What is the correct sequence of these units, beginning with the methane feed?
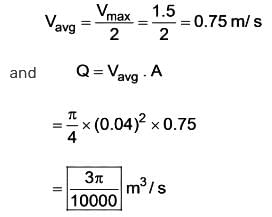
What is the discharge in m3/s for a laminar flow through a pipe with a diameter of 0.04 m, given that the centerline velocity is 1.5 m/s?
The motion x(t) of a particle, moving at a constant ω, is governed by the equation 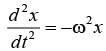 . The initial
. The initial
\nconditions are 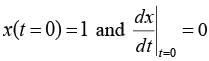 . Determine the position of the particle at t = (3π / ω) __________ (in integer).
. Determine the position of the particle at t = (3π / ω) __________ (in integer).
The total of the Eigenvalues of the matrix  is ______. (round to the nearest integer)
is ______. (round to the nearest integer)
If the capacity of the plant is adjusted to 80%, while maintaining the same annual fixed costs, identical product selling price, and total annual sales of 6,40,000 Rs, what will be the gross profit for the year? (round to the nearest whole rupee). Assume the direct cost per unit is 35 Rs./unit.
An irreversible isomerization reaction of first order is conducted in the liquid phase within a CSTR at a temperature of 165°C. The rate constant at this temperature is 0.7 hr-1. The activation energy required to transform the reactant into the product is 120000 J/mol, and the heat of reaction is –350 kJ/kg. The feed enters the reactor at a volumetric flow rate of 0.33 m3/h and at a feed temperature of 20°C. The specific heat capacity of both the reactants and products is 1.95 kJ/kg-K. The anticipated conversion for this process is 95%. If the reactor functions adiabatically, what will be the temperature of the reaction mixture (in Kelvin)?
Consider a tank filled with liquid where the liquid is entering at a flow rate of Fi while exiting at a flow rate of Fo. The height of the liquid within the tank is represented as h, and the tank has a cross-sectional area of A. The outflow rate is defined by the equation Fo = αh where α is a positive constant. Which of the following represents the root that ensures system stability?
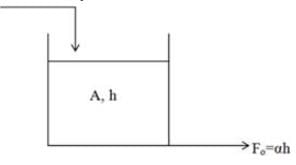
Which of the statements listed below are correct (T) or incorrect (F)?
(A) Both feedback and feedforward control necessitate a measured variable.
(B) In feedback control, the variable that needs to be controlled is measured.
(C) Feedforward control can theoretically be perfect, allowing the controller to act through the manipulated variable while the controlled variable remains at its desired value.
(D) Feedforward control is capable of achieving perfect control; that is, it can maintain the output at its desired level, despite an imperfect process model.
(E) Feedback control will always respond, irrespective of the precision of any process model utilized in its design and the nature of the disturbance.
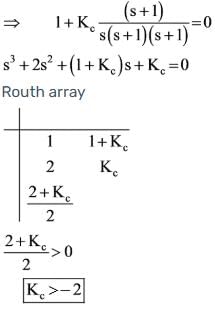
Determine the value of Kc that ensures the stability of the given control system. What will be the corresponding value of x?
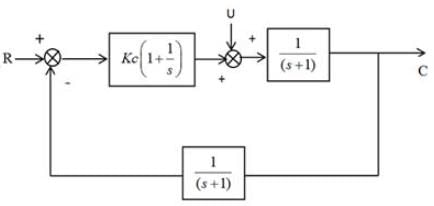
Examine the following assertion about the throttling process of wet steam.
Which of the following statements is/are TRUE?
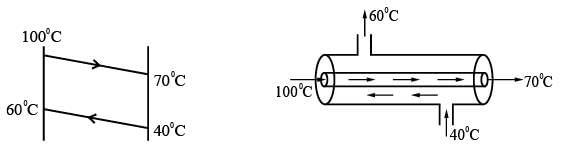
A fluid is heated from 40° C to 60° C using a countercurrent double pipe heat exchanger. The hot fluid enters at 100° C and exits at 70° C. The log mean temperature difference, known as LMTD (in °C), is _______ (round off to 2 decimal places).
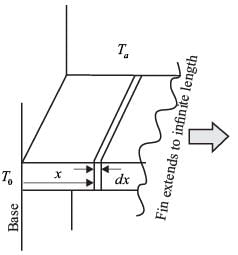
Consider a rectangular fin that extends infinitely and is surrounded by a fluid maintained at a constant temperature of Ta = 27° C.
The steady-state one-dimensional energy balance for a segment of the fin with a thickness of dx, located at a distance x from its base, results in
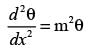
where θ = Ts - Ta, and Tx denotes the temperature of the fin at a distance x from its base, measured in °C. Given that the value of m is 0.04 cm-1 and the base temperature is T0 = 227°C, what is the temperature (in °C) at x = 25 cm, rounded to one decimal place?
Water in liquid form is being pumped at a volumetric flow rate of 0.02 m3s-1 from Tank I to Tank II, as depicted in the diagram.
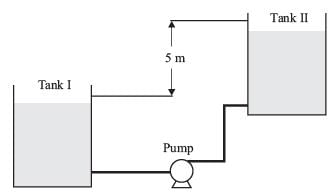
Both tanks are open to atmospheric pressure. The total frictional head loss within the piping system is 1.0 m of water.
Furthermore, consider the following data and assumptions:
- Water density is 1000 kg m-3
- Acceleration due to gravity is 9.81 m s-2
- The pump operates at 100 % efficiency
- The liquid levels in the tanks have negligible velocities
What is the power (in W) delivered by the pump to elevate the water? Please round off your answer to 1 decimal place.
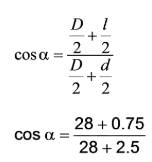
A feed that is similar to spheres with a diameter of 5 cm is processed in a 2 Roll Crusher. When the feed is crushed to a sphere size with a diameter of 1.5 cm and the rolls have a diameter of 56 cm, what will the angle of Nip of the Roll crusher be? (Provide the answer up to one decimal place in degrees). ……………..
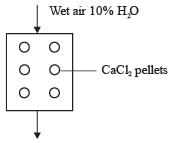
Moist air that has 10 mole percent water vapor is subjected to drying by continuously flowing it through a column filled with CaCl2 pellets. These pellets eliminate 50 percent of the water present in the wet air entering the column. What is the mole percent of water vapor in the product stream that exits the column, rounded to two decimal places?
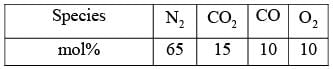
The Orsat analysis, which displays the composition (in mol%, on a dry basis) of a stack gas, is presented in the table below. A measurement of humidity indicates that the mole fraction of H2O in the stack gas is 0.07. What is the mole fraction of N2 calculated on a wet basis, rounded off to two decimal places?
An investment project necessitates an initial capital of 15,000 Rs. It has no salvage value and generates an annual cash inflow of Rs. 5,000. The project's required rate of return is 10% per annum. What will be the net present value rounded to the nearest rupee?
Two different methods, process 1 and process 2, are being evaluated for their effectiveness in lowering steam utility expenses in a petrochemical facility.
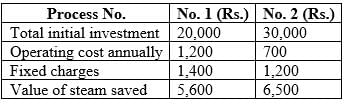
Determine the ratio of the annual percentage return from process 1 to that from process 2.
In the feedback system shown below 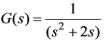 .
.
The step response of the closed-loop system should have minimum settling time and have no overshoot
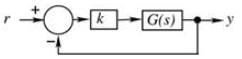
The required value of gain �� to achieve this is ________




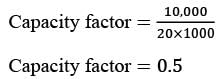
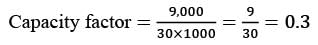
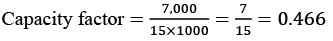
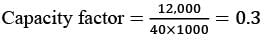

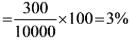
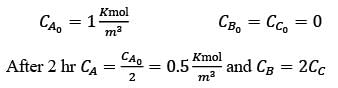

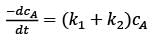
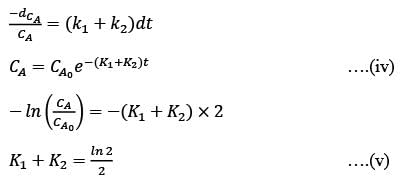

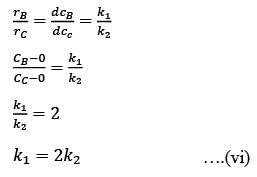
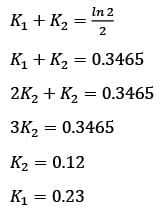
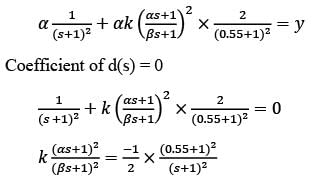
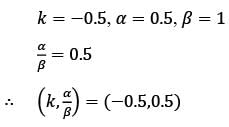
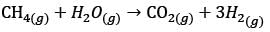
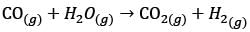
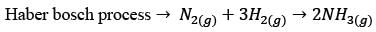
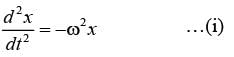
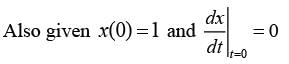

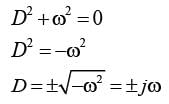
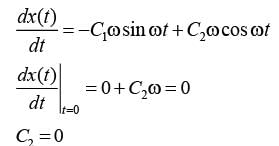
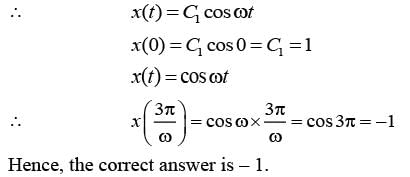
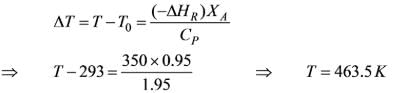
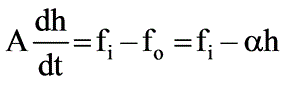
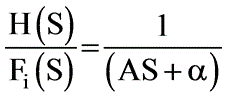
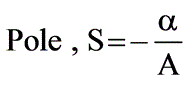
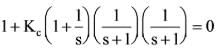
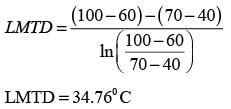
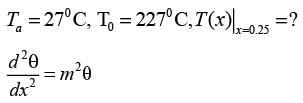
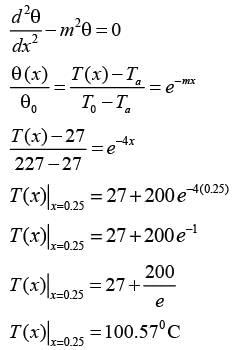
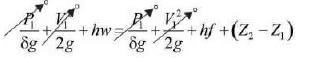

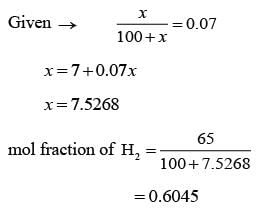
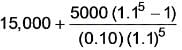 = Rs.3954
= Rs.3954










