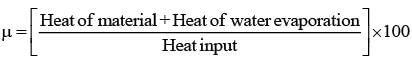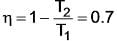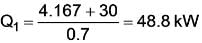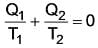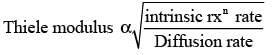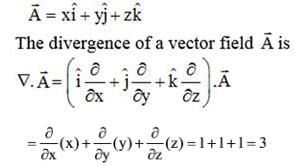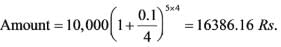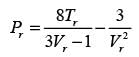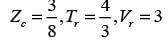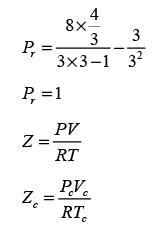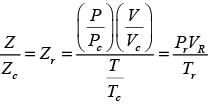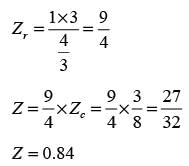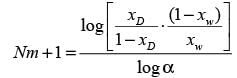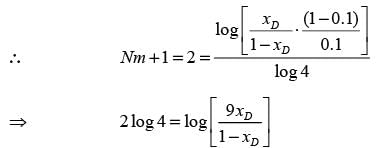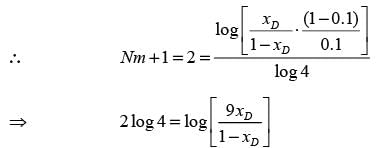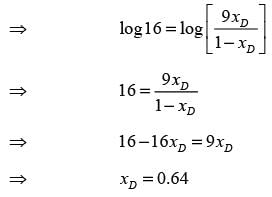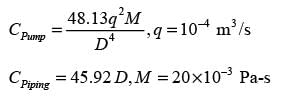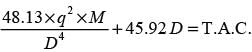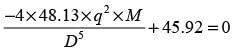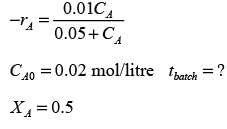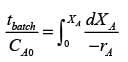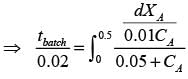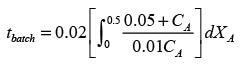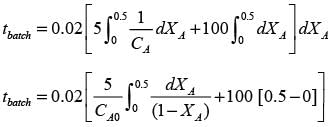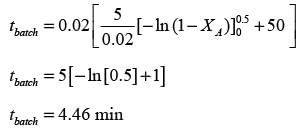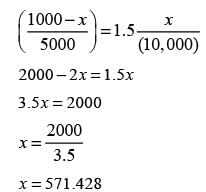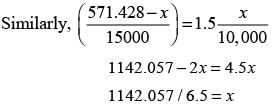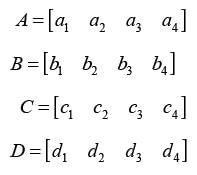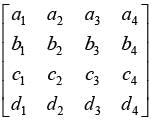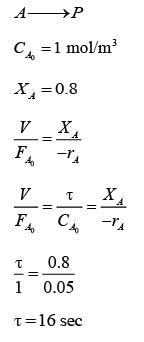GATE Chemical Engineering Mock Test - 7 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 7
Select the sentence that is punctuated correctly:
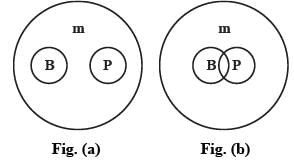
Five individuals (A, B, C, D, and E) are positioned in a row facing north. C is located to the right of B, who is positioned to the left of A. D is seated to the left of C, while E is to the right of A. Who occupies the middle seat?
Some individuals propose implementing Anti-Obesity Measures (AOM), such as showing calorie counts on restaurant menus. These measures, however, fail to confront the fundamental issues leading to obesity, including poverty and income inequality. Which of the following statements best encapsulates the essence of the passage?
Below are two statements labeled 1 and 2, along with two conclusions designated as I and II.
Statement 1: All microorganisms are bacteria.
Statement 2: All microorganisms are pathogens.
Conclusion I: Some bacteria are pathogens.
Conclusion II: Not all bacteria are pathogens.
Considering the statements and conclusions provided, which of the following options is logically accurate?
What is the value of the coefficient of x4 in the expression (x - 1)3 (x - 2)3?
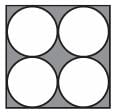
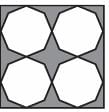
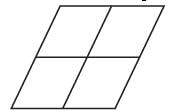
Which of the following shapes can be utilized to completely tile (cover entirely through repetition) a flat surface that extends infinitely in every direction, without leaving any gaps between them? The instances of the shape used in the tiling must be identical and cannot overlap.
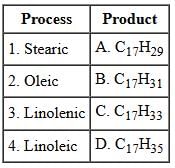
Spray dryers offer numerous benefits. Which of the following is NOT considered an advantage of a standard spray dryer?
In a ball mill, the primary method of size reduction occurs through
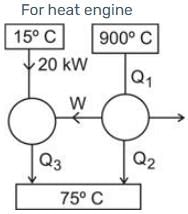
A heat pump extracts heat from a reservoir at 15º C and transfers it to a reservoir at 75º C. This heat pump is powered by a reversible heat engine that absorbs heat from a reservoir at 900º C and expels heat to a reservoir at 75º C. Additionally, the heat engine operates a machine that consumes 30 kW. If the heat pump draws 20 kJ/s from the 15º C reservoir, what is the rate of heat supply from the 900º C source?
The configuration of a power plant is illustrated below.
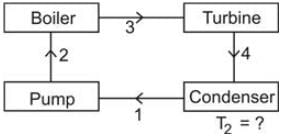
Provided,
h2 = 700 kJ
h3 = 2700 kJ
h4 = 2000 kJ
h1 = 400 kJ
Determine the value of T2(ºC) for which the process is reversible.
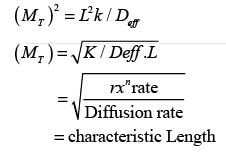
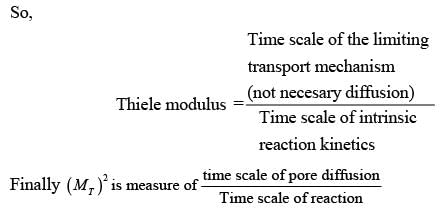
The Thiele modulus squared, MT , is expressed as 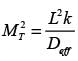 where L denotes the characteristic length of the catalyst pellet, k represents the rate constant for a first-order reaction, and Deff signifies the effective diffusivity of the species within the pores. The term
where L denotes the characteristic length of the catalyst pellet, k represents the rate constant for a first-order reaction, and Deff signifies the effective diffusivity of the species within the pores. The term 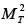 is an indicator of
is an indicator of
What is the divergence of the vector field 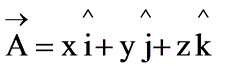 ?
?
Which of the following represents an instance of cationic detergents?
If an amount of Rs. 10,000 is invested at an annual interest rate of 10% compounded quarterly, what will be the value of the investment at the conclusion of 5 years (rounded to 2 decimal places)?
In an isothermal batch reactor, 70% of a liquid reactant is converted within 13 minutes. What is the required space time to achieve this conversion in a plug flow reactor, expressed in minutes?
The van der Waals equation of state can be expressed as follows:
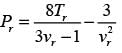
In this equation, Pr, Tr, and vr denote the reduced pressure, reduced temperature, and reduced molar volume, respectively. At the critical point, the compressibility factor (zc) is 3/8. If vr = 3 and Tr = 4/3, what is the value of the compressibility factor derived from the van der Waals equation of state? (Round your answer to two decimal places.)
A distillation column processing a binary mixture of components A and B is functioning at total reflux with two ideal stages, including the reboiler. The mole fraction of the more volatile component in the residue (xW) is 0.1. The average relative volatility αAB is 4. What is the mole fraction of A in the distillate (xD)? (round off to 2 decimal places)
A thick liquid is transported through a network of pipes in a chemical facility. The yearly pumping expense per unit length of the pipe is represented by
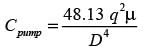
The yearly cost of the installed piping system per unit length is expressed as
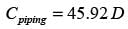
In this context, D signifies the inner diameter of the pipe in meters, q indicates the volumetric flow rate of the liquid in m3s−1, and μ represents the viscosity of the liquid in Pa.s. If the liquid's viscosity is 20 x10−3 Pa.s and the volumetric flow rate is 10−4 m3s−1, what is the optimal inner diameter of the pipe in _______ meters? (round to 3 decimal places)
In a well-stirred batch reactor, reactant A breaks down into products B and C with the aid of an enzyme. The kinetic rate expression is represented as follows:
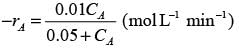
Given that the initial concentration of A is 0.02 mol L−1, calculate the time required to reach a 50% conversion of A, rounding your answer to 2 decimal places.
The system with a closed loop will be categorized as:
What is the definition of the turnover ratio?
A perfect gas weighing one kilogram, initially at 15ºC and 100 kPa, is subjected to heating until it reaches 45ºC through two different processes: (i) one that maintains constant pressure and (ii) one that keeps the volume constant. The specific heat at constant pressure (cp) for the gas is 1.042 kJ/kg.K and the gas constant (R) is 0.2968 kJ/kg.K. Calculate the heat added in both the constant pressure (QP) and constant volume (Qv) processes.
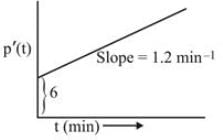
If the input Ym to a PI controller experiences a stepwise change (Ym (s) = 2/s) and the output from the controller alters initially as depicted in the figure, what are the values for the controller gain and the integral time (in min)?
Identify the statements that are correct.
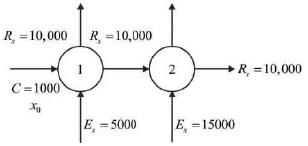
Two ideal counter-current stages are utilized to extract P from a feed that contains both P and Q, as illustrated below.
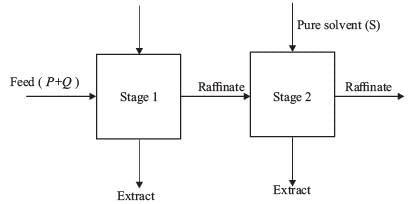
The mass flow rates of P and Q entering stage 1 are 1,000 kg h-1 and 10,000 kg h-1, respectively. Pure solvent (S) is introduced at mass flow rates of 5,000 kg h-1 and 15,000 kg h-1 to stages 1 and 2, respectively. The components Q and S do not mix. The equilibrium relationship is defined by Y* = 1.5X, where X represents the mass of P per unit mass of Q in the raffinate, and Y* denotes the mass of P per unit mass of S in the extract that is in equilibrium with the raffinate. The mass flow rate of P (in kg h-1) in the raffinate from state 2 is ____(round off to nearest integer).
The vectors A, B, C, and D each have a length of 4.
It is given that B is not a scalar multiple of A. Additionally, C is linearly independent of both A and B. Furthermore, D can be expressed as D = 3A 2B C.
Determine the rank of the following matrix:
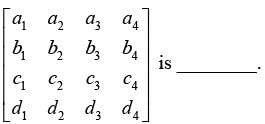
Oil is flowing through a pipe that has a diameter of 0.05 m, with the oil's density being 850 kg/m3. A differential manometer is connected to this pipe, and an orifice plate with a diameter of 0.025 m is inserted as illustrated in the figure.
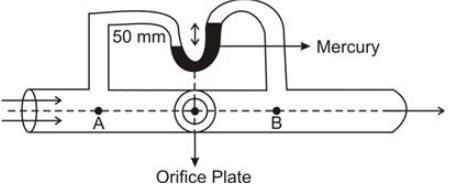
Density of Mercury = 13600 kg/m3
Value of g = 9.81 m/s2
What is the value of PA - PB in kPa? (Provide the answer to three decimal places)
An irreversible unimolecular reaction in the liquid phase, represented as
A → products, was conducted in an ideal batch reactor at a temperature T. The reaction rate (-rA) at various conversions XA is provided in the table below. This reaction was also performed in an ideal continuous stirred tank reactor (CSTR) at the same temperature T with a feed concentration of 1 mol.m-3, maintaining steady-state conditions. For a conversion of 0.8, what is the space time (in seconds) of the CSTR, expressed as an integer?

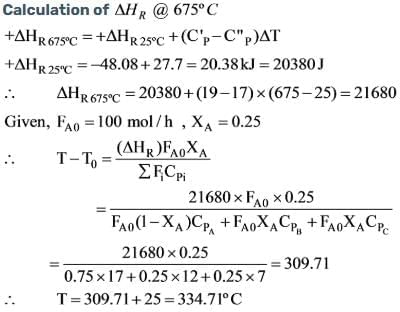
Methanol is introduced into an adiabatic reactor at 675oC and 1 bar, at a flow rate of 100 mol/h, where 25% of it undergoes dehydrogenation to produce formaldehyde according to the reaction shown below.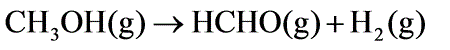
Determine the temperature of the gases exiting the reactor, assuming the average heat capacities remain constant at 17, 12, and 7 J/mol ºC for methanol, formaldehyde, and hydrogen, respectively. (Neglect any changes in kinetic and potential energy).
The standard heats of formation at 25ºC in kJ/mol are provided as follows: