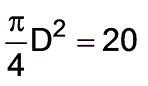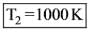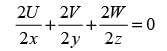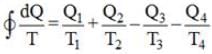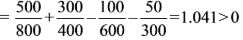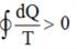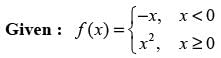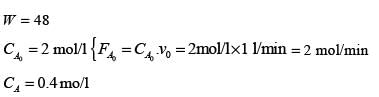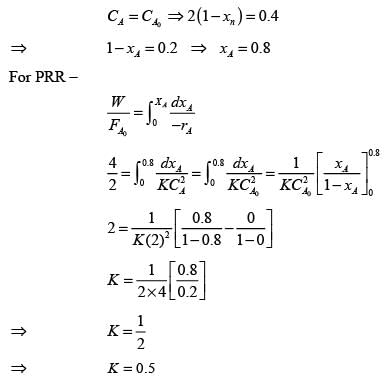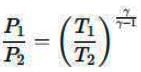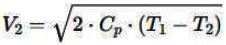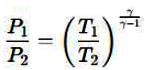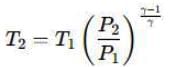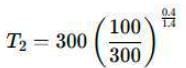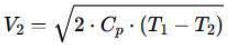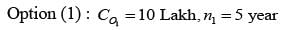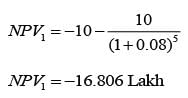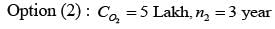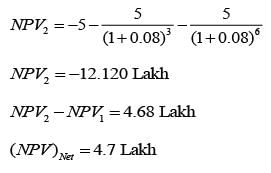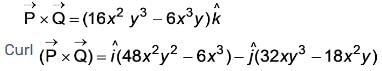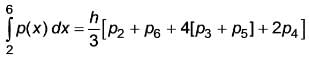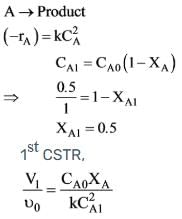GATE Chemical Engineering Mock Test - 8 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 8
Which of the following statements is accurate regarding grammar and usage?
It is extremely generous of you to handle the washing-up, but you ________ it.
It is extremely generous of you to handle the washing-up, but you ________ it.
In spite of a series of disappointing performances, the likelihood of K.L. Rahul being chosen for the team is ___.
Choose the term that corresponds with the analogy:
Cover : Uncover : : Associate : ____
Cover : Uncover : : Associate : ____
Ravi leaves his home and walks 10 km to the North. He then makes a right turn and walks 5 km. After that, he turns right once more and walks 10 km. Finally, he turns left and walks 10 km. What direction is Ravi in now relative to his starting point?
At what moment between 4:00 and 5:00 will the hands of a clock form a right angle?
Which of the following statements is accurate?
A total of 116 J of work needs to be applied isobarically to a rod within a piston cylinder that operates at a pressure of 101.325 kPa. What diameter of the cylinder is required to limit the rod's movement to a maximum of 0.5 m? Assume the rod has a mass of 3 kg.
The temperature range for the Haber process operates between 350 and 500°C. This process is utilized for producing ammonia at
For which conditions is the ideal gas law applicable?
The function f(z) = (z - 1) / (z² 1) exhibits singularities at which points?
In the oxidation-reduction process of H2S for the production of elemental sulfur, which catalyst is utilized?
Which of the following is NOT an essential requirement for maintaining stability in a closed-loop control process?
Ethylene attaches to the available active sites V of a transition metal catalyst through the following mechanism.
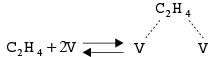
If NT, NV, and NC2H4 represent the total number of active sites, the number of unoccupied active sites, and the number of C2H4 molecules that are adsorbed, respectively, the equation governing the total number of active sites can be expressed as
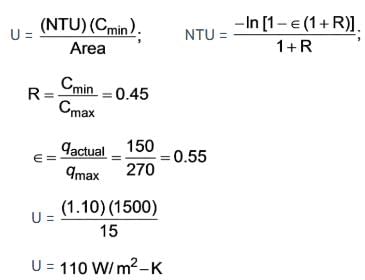
In a double pipe heat exchanger, water is moving at a rate of 0.80 kg/sec with a specific heat capacity of 4197 J/kg·K. A gas enters the system at a flow rate of 1.50 kg/sec and a temperature of 320°C. The water is heated from 50°C to 150°C by the gas.
Details: Specific heat capacity of the gas = 1000 J/kg·K,
Gas inlet temperature = 320°C,
Gas outlet temperature = 170°C,
This is a parallel flow heat exchanger with an area of 15 m2. Determine the overall heat transfer coefficient.
A black body radiates energy at a peak wavelength of 1.45 μm when its temperature is 2000K. If this peak wavelength shifts to 2.90 μm, what will be the new temperature (in K) of the black body?
Which of the following statements is incorrect?
A velocity field in three dimensions is expressed as V = 5x2 yi Cyj - 10 xyz k, where i, j, k denote the unit vectors in the x, y, and z directions, respectively, in a Cartesian coordinate system. The constant C is a fixed value. If the velocity field V characterizes an incompressible fluid flow, what is the value of C?
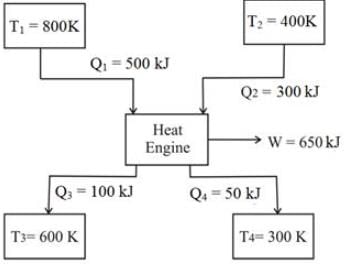
An inventor asserts that he has created a device that operates cyclically, absorbing 500 kJ and 300 kJ of heat from heat reservoirs at temperatures of 800 K and 400 K, respectively. It also rejects 100 kJ and 50 kJ of heat to reservoirs at 600 K and 300 K, respectively, while producing 650 kJ of work. Do you find his assertion to be valid?
The vapor pressure of liquid ammonia (in atmospheres) around the triple point can be represented as:
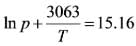
where the temperature T is given in Kelvin.
Similarly, the vapor pressure of solid ammonia can be described by
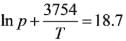
Assume the molecular mass of ammonia is 17 kg / kmol.
What are the temperature and pressure at the triple point?
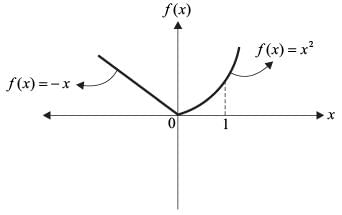
For the function 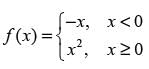
which of the following statement(s) is/are CORRECT?
A catalytic gas phase reaction P → Q takes place in an isothermal packed bed reactor that is maintained at a steady state. The reaction is irreversible and follows second-order kinetics with respect to the reactant P. The feed consists solely of P, with a volumetric flow rate of 1.0 liter minute-1 and a concentration of 2.0 mol liter-1. Additionally, consider the following assumptions:
- The reactant and the product behave as ideal gases.
- No volume change occurs as a result of the reaction.
- Ideal plug flow conditions are present in the packed bed.
When the mass of catalyst within the reactor is 4 g, the concentration of P observed at the exit is 0.4 mol liter-1. Calculate the second-order rate constant (in liter2 g-1catalyst mol-1 minute-1) to one decimal place.
Air is moving through a converging nozzle with an inlet pressure of 300 kPa and an inlet temperature of 300 K. The air can be considered as an ideal gas with a specific heat ratio (γ) of 1.4. If the pressure at the nozzle exit is 100 kPa, determine the exit velocity in m/s of the air at the exit of the nozzle.
Given:
Inlet pressure, P1 = 300 kPa
Inlet temperature, T1 = 300 K
Exit pressure, P2 = 100 kPa
Specific heat ratio, γ = 1.4
In the case of a lower triangular matrix, the product of the eigenvalues is represented as 'P', where 'P' can be any number, including zero. The total of the eigenvalues is denoted as 'D'. What will be the total of the cubes of the eigenvalues, given that one of the eigenvalues is '0' (Zero)?
A design engineer is required to acquire a membrane module (M) for a facility. The specifications for the two available alternatives, M1 and M2, are presented in the table below. The anticipated lifespan of the overall plant is 7 years. Given an interest rate of 8% per annum, compounded annually, what is the difference in the net present value (NPV) between these two alternatives, expressed in lakhs of rupees, rounded to one decimal place?

If
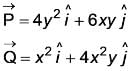
The curl of the vector 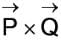 is represented as
is represented as 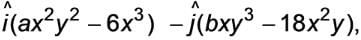 where
where 
 are the vectors pointing in the x and y directions, respectively. What is the value of a/b. (To two decimal places).
are the vectors pointing in the x and y directions, respectively. What is the value of a/b. (To two decimal places).
A matrix A of dimensions n × n is provided as follows:
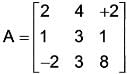
Determine the value of | Adj. A |.
Given the values of p(x) for the interval [2, 6].
Applying the Simpson's rule 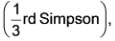 , what is the result of
, what is the result of 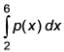 ? (Round off to two decimal places) _______
? (Round off to two decimal places) _______
The function q(x) = 2x2 - 2.5x 12 fulfills the conditions of the Lagrange mean value theorem for the interval (2, 6) at the value of x equal to _______
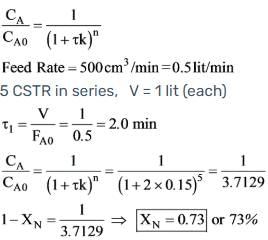
A liquid reactant stream with CAo = 1 mol/litre flows through two mixed flow reactors arranged in series. The concentration of 'A' in the exit stream from the first reactor is 0.5 mol/lit. What will the concentration of 'A' be in the exit stream of the second reactor? The reaction A → R follows second order kinetics and V2/V1 = 2.
In a solution of water, the reaction P → Q takes place under isothermal conditions and follows first order kinetics. The feed rate is set at 500 cm3/min, with the concentration of P in the feed being 1.5 x 10-4 mol/cm3. The existing 5 litre CSTR is substituted with five CSTRs arranged in series, each having a capacity of 1 litre. Given that the rate constant K is 0.15 min-1, what will be the overall percentage conversion?