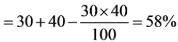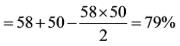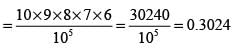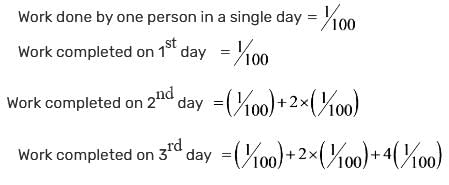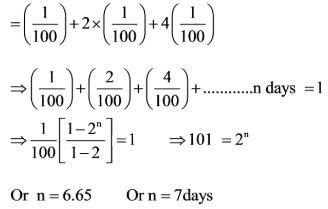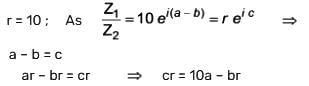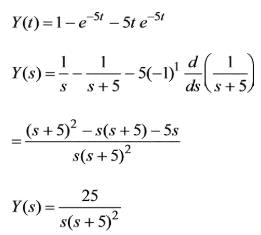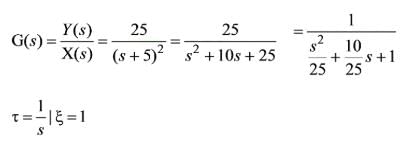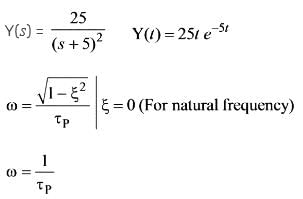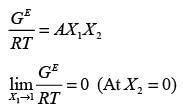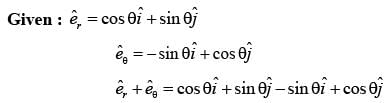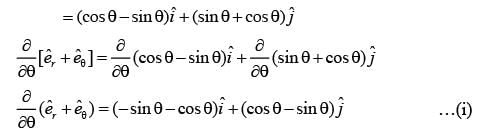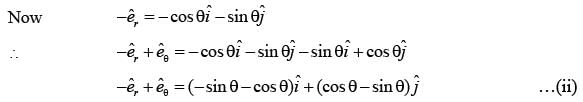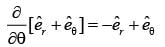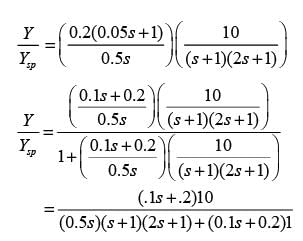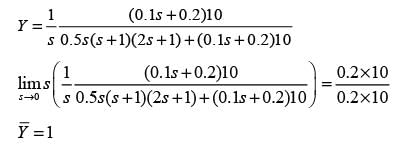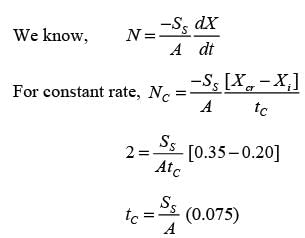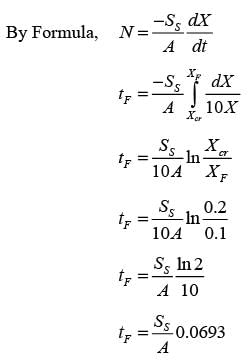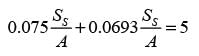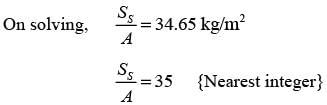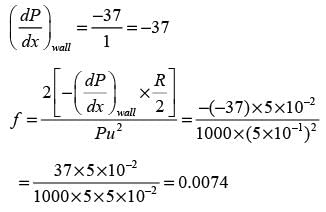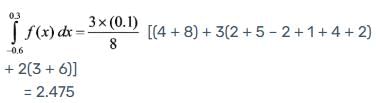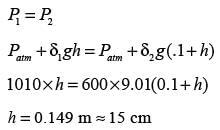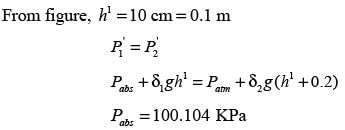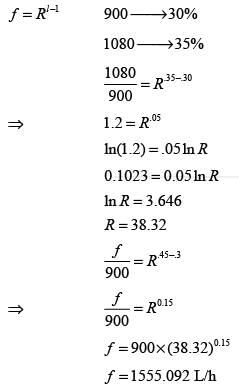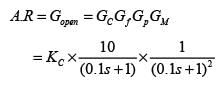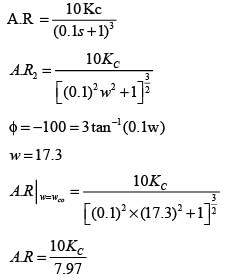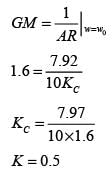GATE Chemical Engineering Mock Test - 9 - GATE Chemical Engineering MCQ
30 Questions MCQ Test - GATE Chemical Engineering Mock Test - 9
Determine a single discount that is equivalent to applying three consecutive discounts of 30%, 40%, and 50%.
_____ relates to surgery in the same way that a writer relates to _____. Which of the following options preserves a similar logical connection in the statement above?
There are five bags, each containing identical sets of ten different chocolates. If one chocolate is selected from each bag, what is the probability that at least two of the chocolates selected are the same?
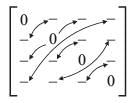
Consider a matrix M = [mij]; where i, j = 1, 2, 3, 4, with all diagonal elements being zero and the property that mij = -mji. What is the minimum number of elements needed to completely define the matrix?
A certain group of individuals is capable of finishing a specific task in 100 days if they work alone. On the first day, one individual works; on the second day, two additional individuals join; on the third day, four more individuals join; and this pattern continues until the task is completed. How many days will it take to finish the task?
You are given two complex numbers as follows:

What is the value of 'Cr'?
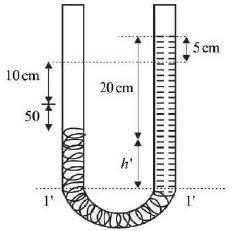
Both ends of the manometer are open to the atmosphere.
ρ oil₁ – density of oil 1
ρ oil₂ – density of oil 2
h₁ and h₂ – respective heights in cm.
Choose the set which satisfies the data of the diagram:
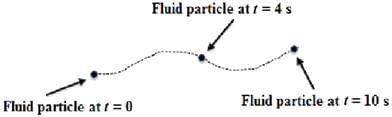
In an experiment, the position of a fluid particle is tracked by a device over a duration of 10 seconds. The path of the particle illustrated in the figure represents a
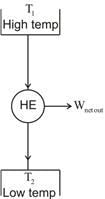
A heat engine is defined as a device that -
Which of the following options correctly explains the term “Continuum”?
A system of second order is characterized by the given equation. What are the respective values of the frequency and damping ratio?
The unit step response of a second-order system is expressed as 1 - e-5t - 5te-5t. Examine the following assertions:
1. The undamped natural frequency is 5 rad/s.
2. The damping ratio equals 1.
3. The impulse response is defined.
Which of the above assertions are accurate?
In a binary liquid mixture, the mole fraction of component 1 is denoted as x1, and its activity coefficient is represented by γ1. The excess molar Gibbs energy of the mixture is indicated as GE, while R stands for the universal gas constant and T represents the absolute temperature of the mixture. Which of the following statements is always correct?
In a two-dimensional plane, the unit vectors, 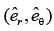 of the polar coordinate system and
of the polar coordinate system and 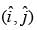 of the Cartesian coordinate system, are connected through the following two equations.
of the Cartesian coordinate system, are connected through the following two equations.
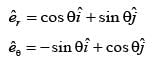
Which of the following represents the CORRECT value of 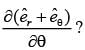 ?
?
Which of the following statements concerning the octane number is NOT accurate?
Examine the refining processes presented in Group-I alongside the catalysts outlined in Group-II.
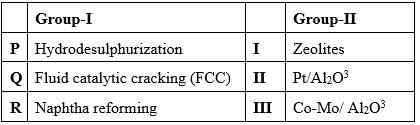
What is the appropriate pairing?
Analyze the procedures in Group - 1 alongside the reactions occurring in Group - 2
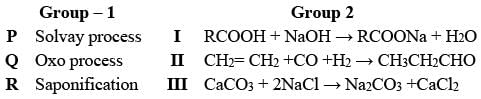
What is the accurate combination?
Examine the following closed-loop system where Gp and Gc represent the transfer functions of the process and the controller, respectively.
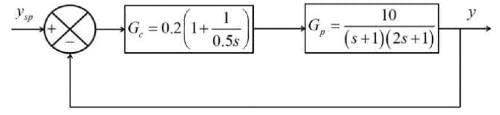
For a unit step alteration in the set point ( ysp), what is the change in the steady-state value of the response ( y )? Please provide your answer rounded to one decimal place.
A mixture of gases at a pressure of 1 bar and a temperature of 300 K contains 20 mol % CO2 and 80 mol % an inert gas. Assume the gases behave ideally. Given R = 8.314 J mol-1K-1, what is the minimum amount of work, in kJ, required to separate 100 mol of this mixture at 1 bar and 300 K into pure CO2 and the inert gas, maintaining the same temperature and pressure? (round off to the nearest integer)
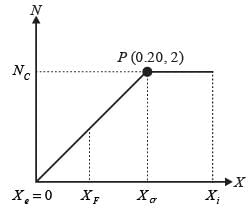
In an experiment involving batch drying, a solid with a critical moisture content of 0.2 kg H2O/kg dry solid is reduced from an initial moisture content of 0.35 kg H2O/kg dry solid to a final moisture content of 0.1 kg H2O/kg dry solid over a period of 5 hours. During the constant rate phase, the drying rate is 2 kg H2O/(m2h). The entire falling rate phase is assumed to follow a uniformly linear pattern. The equilibrium moisture content is considered to be zero. What is the mass of the dry solid per unit area in kg/m2? (round off to the nearest integer)
It is necessary to manage the volume of the substances in the jacketed reactor depicted in the figure.
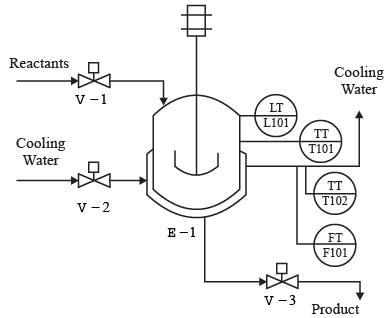
Which of the following schemes is suitable for implementing feedback control?
Water with a density of 1000 kg m−3 flows through a horizontal pipe that has a diameter of 10 cm, moving at an average speed of 0.5 ms−1. The following graph illustrates the pressure readings taken at various points along the pipe from its entrance.
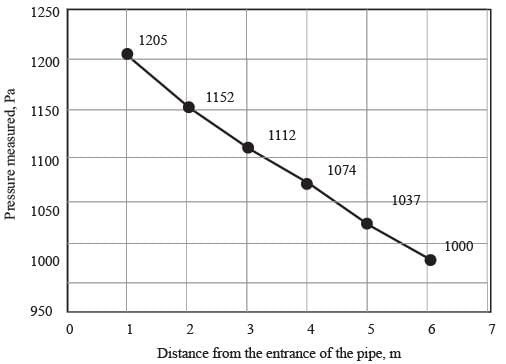
Based on the information presented in the figure, determine the Fanning friction factor in the pipe under conditions of FULLY DEVELOPED flow.
Employing Simpson's 3/8 rule, calculate the value of the integral given the following data.
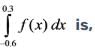
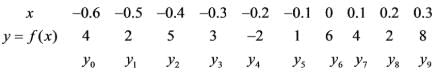
The operating conditions of steam at the entrance and exit of a perfectly insulated steam turbine functioning under steady-state conditions are detailed below:
At the inlet: specific enthalpy = 3230 kJ/kg; velocity = 160 m/s
At the outlet: specific enthalpy = 2660 kJ/kg; velocity = 100 m/s
Ignoring any variations in potential energy, what is the approximate work output of the steam turbine?
A U-tube manometer is filled with two different manometric fluids, having densities of 1000 kg m−3 and 600 kg m−3. When both arms of the manometer are open to the atmosphere, the height difference between the two fluid levels is 10 cm at equilibrium, as illustrated in the figure.
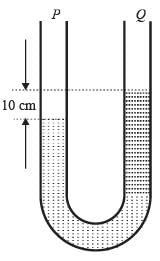
The remaining section of the manometer is occupied by air with negligible density. With an acceleration due to gravity of 9.81 m s−2 and atmospheric pressure at 100 kPa, what absolute pressure (in kPa) must be exerted on limb ‘P’ to elevate the fluid in limb ‘Q’ by an additional 20 cm?
The flow rate of water through an equal percentage valve is 900 liters h-1 when the valve is opened to 30%, and 1080 liters h-1 at a 35% opening. Assuming that the pressure drop across the valve remains unchanged, what is the flow rate (in liters h-1) through the valve at a 45% opening? Please round your answer to the nearest integer.
Examine the following closed loop system.
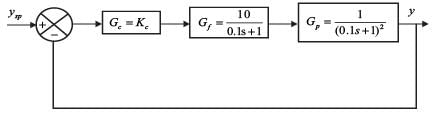
Gc, Gf, and Gp represent the transfer functions of the controller, the final control element, and the process, respectively. y and ysp denote the response and its set point, respectively. Given a gain margin of 1.6, what is the design value of KC _____ (rounded to one decimal place)?
The compressibility factor for a gas is defined as
Z = 1 B.P
Calculate the volume Z = 1 [in m3] of 1 mole of a real gas, given that the volume of 1 mole of the gas is 0.04 m3 when it behaves ideally. The temperature is provided as 50º C.
B = 2.234 x 10-5 Pa-1
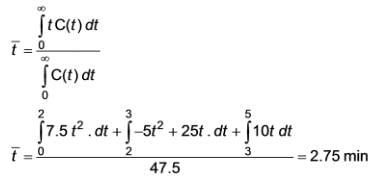
In a Continuous Stirred Tank Reactor (CSTR), the concentration of a tracer is recorded over time, as illustrated in the plot below:
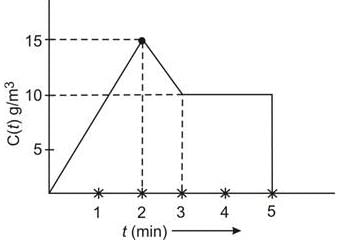
Determine the mean residence time in minutes. (To two decimal places) ……………………….
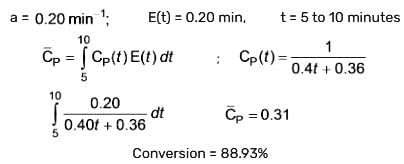
The residence time distribution for a reactor is illustrated in the figure below:
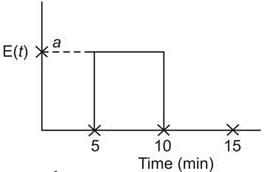
A second-order reaction is occurring in the reactor system, represented as P → Q, where the feed concentration of P is 2.8 mol/litre and the rate constant is 0.4 L/mol.min. What is the conversion percentage of P (rounded to two decimal places)?