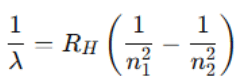J.J. Thomson (1897) discovered electrons using the Cathode Ray Tube experiment.
He applied high voltage to a glass tube and observed rays moving from cathode to anode.
The rays were deflected by electric and magnetic fields, showing they were negatively charged particles present in all atoms—later named electrons.
|
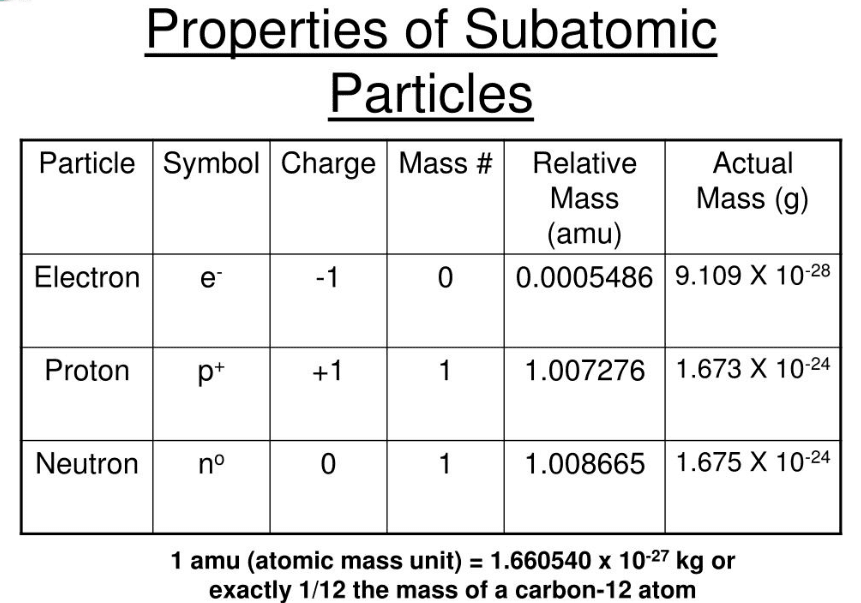
Atoms are composed of three main subatomic particles: 1. Electron (e-) 2. Proton (p+) 3. Neutron (no)
|
Card: 2 / 30 |
|
|
Card: 4 / 30 |
|
J.J. Thomson used electric and magnetic fields in a Cathode Ray Tube experiment to determine e/m ratio: e/m = 1.7588820 × 1011 C/kg |
Card: 6 / 30 |
|
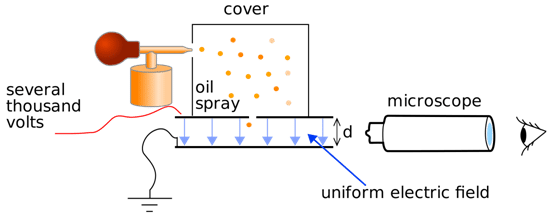
R.A. Millikan (1906–1914) determined the electron’s charge (-1.602 × 10⁻¹⁹ C) using the Oil Drop Experiment.
|
Card: 8 / 30 |
|
Proton (p+): Ernest Rutherford (1919) through Gold Foil Experiment. Neutron (no): James Chadwick (1932) by bombarding beryllium with alpha particles. |
Card: 10 / 30 |
|
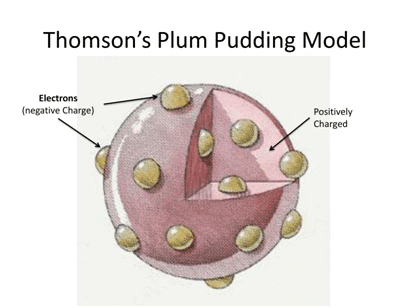
Proposed by J.J. Thomson (1898), the atom was seen as a positively charged sphere with embedded electrons, like ‘plums in pudding’.
|
Card: 12 / 30 |
|
Rutherford bombarded a thin gold foil with alpha particles (He2+ ions). He concluded:
|
Card: 14 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Niels Bohr (1913) proposed that:
|
Card: 16 / 30 |
|
c = λv
|
Card: 18 / 30 |
|
Max Planck (1900) proposed that energy is quantized: E = hv
|
Card: 20 / 30 |
|
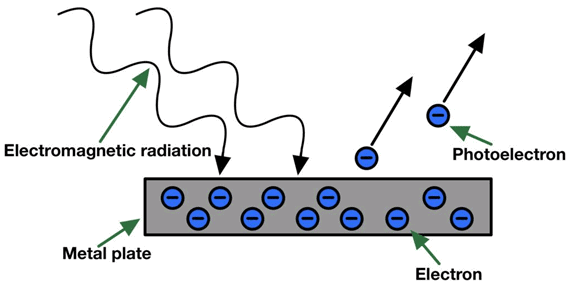
Albert Einstein explained the Photoelectric Effect using quantum theory: KE = hv - hvo
|
Card: 22 / 30 |
|
1. Principal Quantum Number (n): Energy level (n = 1, 2, 3, ...) 2. Azimuthal Quantum Number (l): Shape of orbital (s = 0, p = 1, d = 2, f = 3) 3. Magnetic Quantum Number (m1): Orientation of orbital (-l to +l) 4. Spin Quantum Number (mS): Electron spin (+1/2 or -1/2) |
Card: 24 / 30 |
|
It is impossible to know both position and momentum of an electron simultaneously: Δx.Δp ≥ h/4π
|
Card: 26 / 30 |
|
Where:
|
Card: 30 / 30 |