|
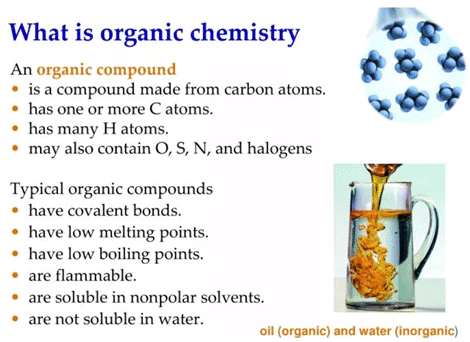
Organic chemistry is the study of carbon-containing compounds, including hydrocarbons and their derivatives. It focuses on the structure, properties, reactions, and applications of organic molecules. |
Card: 2 / 42 |
|
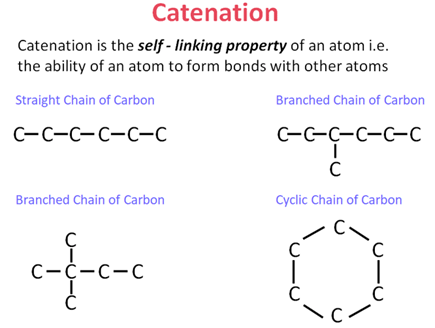
Catenation is the ability of carbon atoms to form long chains and rings by bonding with other carbon atoms. This property allows the formation of a vast number of organic compounds. |
Card: 4 / 42 |
|
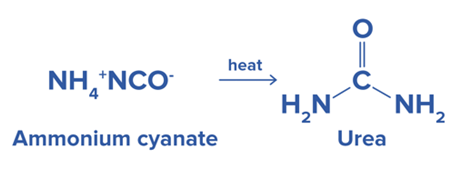
Urea (NH2CONH2) was the first organic compound synthesized by Friedrich Wöhler in 1828 from ammonium cyanate, proving that organic compounds can be synthesized from inorganic. |
Card: 6 / 42 |
|
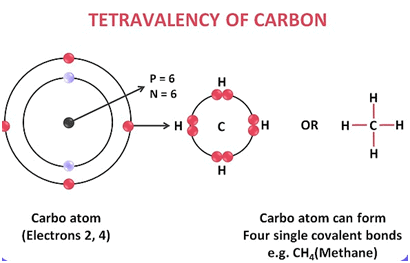
Carbon has four valence electrons and forms four covalent bonds to complete its octet, making it tetravalent. |
Card: 8 / 42 |
|
Card: 10 / 42 |
|
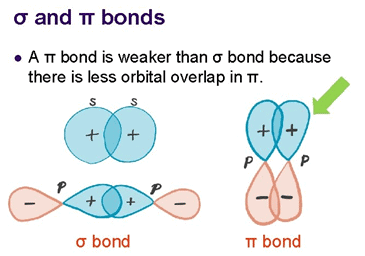
π-bonds are formed by sideways overlap of p-orbitals and have electron density above and below the plane of the molecule, making them more accessible to attacking reagents. |
Card: 12 / 42 |
|
Acyclic (Open-chain) Compounds – Straight or branched-chain structures (e.g., alkanes, alkenes, alkynes). Cyclic (Closed-chain) Compounds – Carbon atoms form a ring.
|
Card: 14 / 42 |
|
A functional group is an atom or group of atoms in a molecule responsible for its chemical properties. |
Card: 16 / 42 |
|
-OH (Hydroxyl) → Alcohols -CHO (Aldehyde) → Aldehydes >C=O (Carbonyl) → Ketones -COOH (Carboxyl) → Carboxylic acids -NH₂ (Amino) → Amines |
Card: 18 / 42 |
|
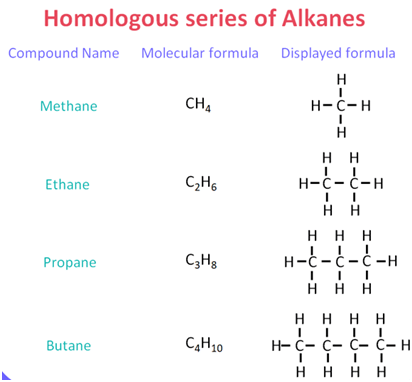
A homologous series is a group of organic compounds with the same general formula, similar chemical properties, and a difference of CH2 in molecular formula between consecutive members. |
Card: 20 / 42 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Identify the longest carbon chain (parent chain). Number the carbon chain to give lowest numbers to substituents. Identify and name the functional groups and substituents. Arrange substituent names alphabetically and use prefixes (di-, tri-, etc., for multiple identical groups). |
Card: 22 / 42 |
|
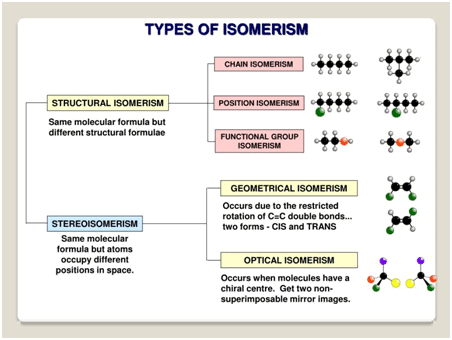
Isomerism is the phenomenon where compounds have the same molecular formula but different structures or properties. |
Card: 26 / 42 |
|
Structural Isomerism (Different connectivity)
Stereoisomerism (Same connectivity, different spatial arrangement)
|
Card: 28 / 42 |
|
Homolytic Cleavage → Each atom takes one electron (forms radicals). Heterolytic Cleavage → One atom takes both electrons (forms ions). |
Card: 30 / 42 |
|
Nucleophiles (electron-rich) → Donate electron pairs (e.g., OH⁻, NH₃, CN⁻). Electrophiles (electron-deficient) → Accept electron pairs (e.g., H⁺, NO₂⁺, BF₃). |
Card: 32 / 42 |
|
Card: 34 / 42 |
|
Card: 36 / 42 |
|
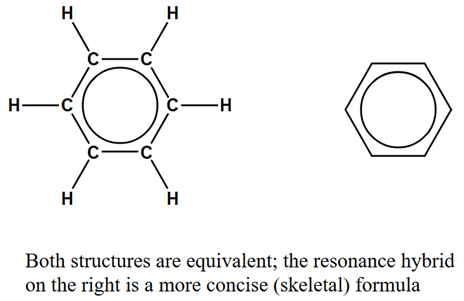
Resonance occurs when a molecule has two or more valid Lewis structures, leading to electron delocalization and stability. Example: Benzene (C6H6). |
Card: 38 / 42 |
|
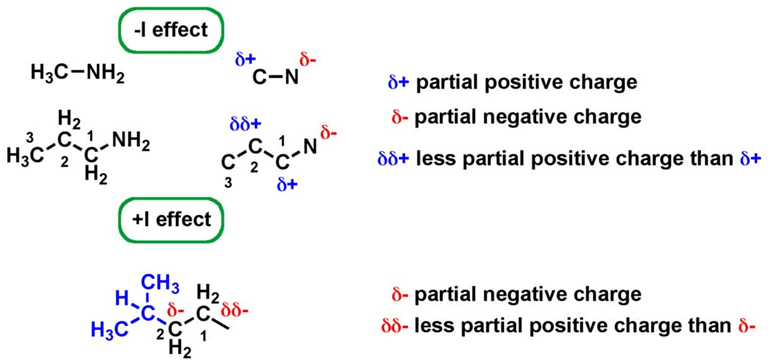
It is the electron withdrawal or donation through sigma bonds due to electronegativity differences. |
Card: 40 / 42 |
|
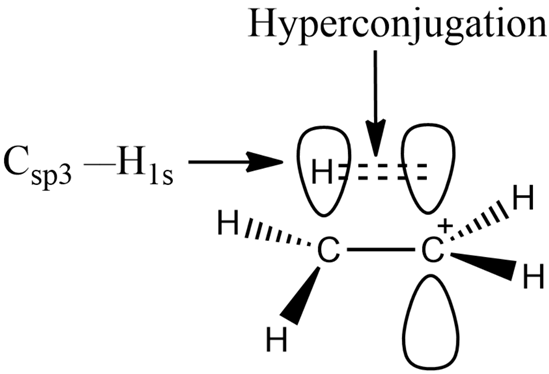
It is the delocalization of electrons from a C-H bond adjacent to a double bond or carbocation, increasing stability. |
Card: 42 / 42 |