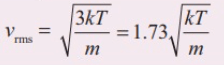|
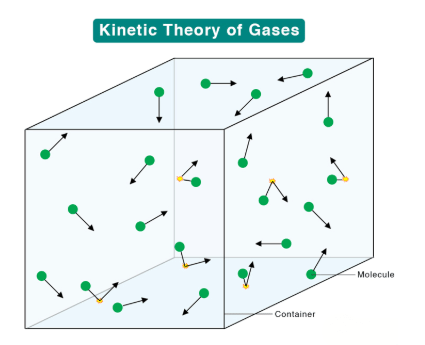
It explains that gases consist of rapidly moving atoms or molecules, and their behavior can be understood in terms of molecular motion and interactions.
|
Card: 2 / 28 |
|
Is the following statement True or False? 'The average distance between gas molecules is smaller than the interatomic distance in solids.' |
Card: 3 / 28 |
|
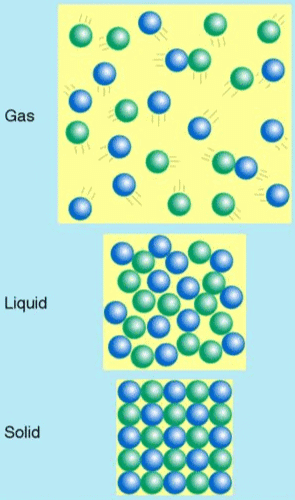
False. The average distance between gas molecules is larger than the interatomic distance in solids.
|
Card: 4 / 28 |
|
Riddle: I am a very small particle, yet I am the building block of everything. What am I? |
Card: 5 / 28 |
|
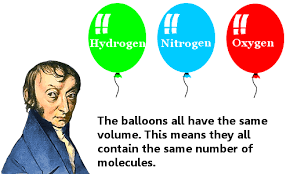
It states that equal volumes of all gases at the same temperature and pressure contain the same number of molecules.
|
Card: 8 / 28 |
|
Fill in the blank: The mean free path is the average distance a molecule can travel without ___ . |
Card: 9 / 28 |
|
Which of the following gases would have the highest root mean square speed at the same temperature? (a) Oxygen (b) Nitrogen (c) Helium (d) Argon |
Card: 11 / 28 |
|
True or False: The pressure exerted by a gas is the same at all points within the gas when in equilibrium. |
Card: 13 / 28 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
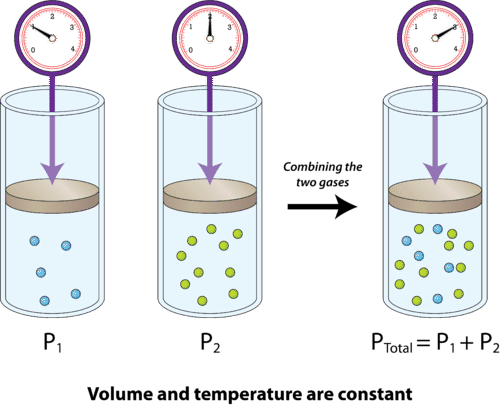
It states that the total pressure of a mixture of non-reactive gases is equal to the sum of the partial pressures of each individual gas.
|
Card: 16 / 28 |
|
Fill in the blank: The average kinetic energy of a molecule in a gas is directly proportional to its absolute ___ . |
Card: 17 / 28 |
|
Riddle: I change with temperature, but my total energy is always conserved. What am I? |
Card: 19 / 28 |
|
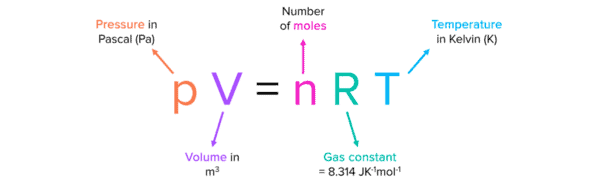
It relates the pressure (P), volume (V), and temperature (T) of a gas, expressed as PV = nRT, where n is the number of moles.
|
Card: 22 / 28 |
|
Fill in the blank: The law of equipartition of energy states that the energy for each degree of freedom is ___ kB T. |
Card: 23 / 28 |
|
True or False: An ideal gas is a theoretical model that behaves exactly like real gases under all conditions. |
Card: 25 / 28 |