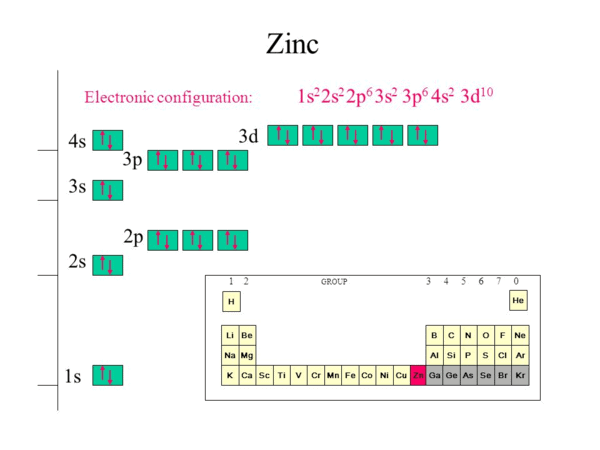
|
Fill in the blanks: d-Block elements are characterized by the presence of ___ electrons in their ___ orbitals. |
Card: 1 / 30 |
f-Block ElementsDistinction Between f-Block and d-Block Elements
|
Card: 4 / 30 |
|
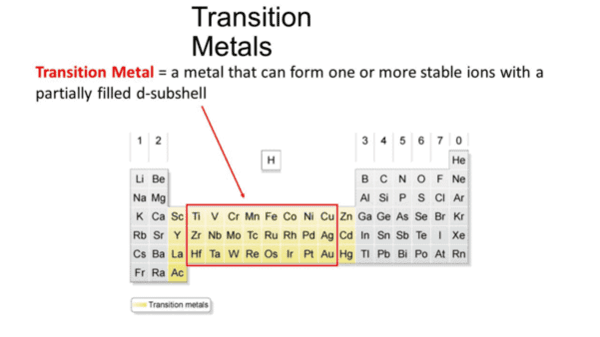
True or False: Transition elements are defined as elements with completely filled d-orbitals. |
Card: 5 / 30 |
|
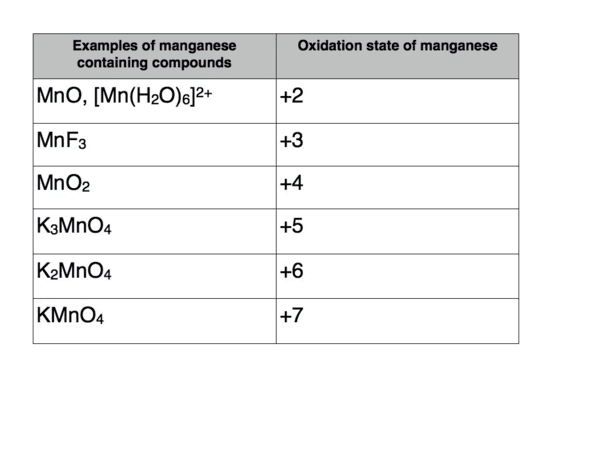
Which d-Block element has the most variable oxidation states and what are the ranges of those states? |
Card: 7 / 30 |
|
What is the primary reason for the high melting and boiling points of d-Block elements? |
Card: 9 / 30 |
Strong Metallic BondingThe Properties of d-Block Elements
|
Card: 10 / 30 |
|
Riddle: I am a metal that can exist in multiple oxidation states, and my highest state is +7. Who am I? |
Card: 11 / 30 |
|
Fill in the blank: Potassium dichromate is a strong oxidizing agent in ___ medium. |
Card: 13 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
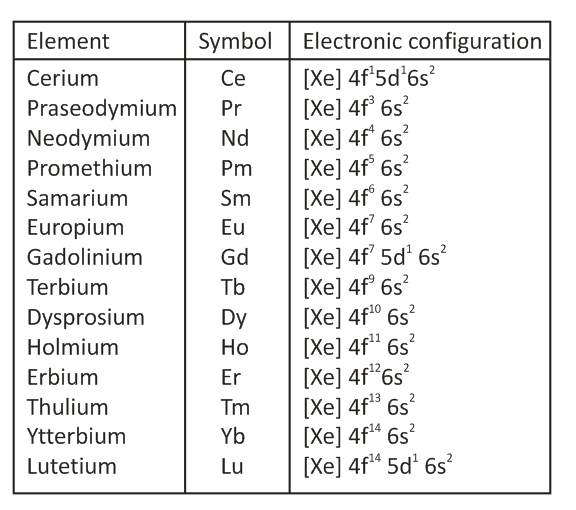
True or False: Lanthanoid contraction refers to the decrease in atomic size across the f-Block elements due to increased shielding by 4f electrons. |
Card: 19 / 30 |
FalseUnderstanding Lanthanoid Contraction
|
Card: 20 / 30 |
|
Which d-Block element is an exception for having a filled d-orbital in its common oxidation state and what is that state? |
Card: 23 / 30 |
|
Riddle: I am known for my purple color and strong oxidizing capabilities in any medium. What am I? |
Card: 25 / 30 |
|
What is the general trend of ionization enthalpies across the d-Block elements? |
Card: 27 / 30 |
Ionization EnthalpiesTrends in d-Block Elements
|
Card: 28 / 30 |
|
The color is due to d-d or f-f electronic transitions in partially filled orbitals.  |
Card: 30 / 30 |