|
The rate of reaction can be quantified by measuring the ___ formed or ___ used up in a given time period. |
Card: 1 / 50 |
|
True or False: The production of hydrogen gas in the reaction between magnesium ribbon and dilute sulfuric acid indicates that a reaction is occurring. |
Card: 3 / 50 |
|
In the magnesium-sulfuric acid reaction, how does the rate of hydrogen gas production change over time? |
Card: 5 / 50 |
|
It starts rapidly but slows down and eventually stops as the reaction progresses.  |
Card: 6 / 50 |
|
Fill in the blank: Measuring the ___ of the flask every 30 seconds during the reaction between calcium carbonate and hydrochloric acid shows a rapid decrease in mass. |
Card: 7 / 50 |
Graphs Plotting Product Formed or Reactant ConsumedAnalyzing Reaction Rates
|
Card: 10 / 50 |
|
Riddle: I am produced when magnesium meets sulfuric acid, and I escape as bubbles. What am I? |
Card: 11 / 50 |
|
True or False: The rate of reaction remains constant throughout the entire process. |
Card: 13 / 50 |
|
False; the rate of reaction changes as the reactants are consumed and products are formed.  |
Card: 14 / 50 |
|
Fill in the blank: A syringe attached to a flask can be used to collect ___ gas during the magnesium-sulfuric acid reaction. |
Card: 15 / 50 |
|
Short Answer: What happens to the volume of gas produced as the reaction between magnesium and sulfuric acid progresses? |
Card: 17 / 50 |
|
The volume of gas produced initially increases rapidly, then slows down before stopping.  |
Card: 18 / 50 |
|
What indicates that a reaction is taking place in the reaction between calcium carbonate and hydrochloric acid? |
Card: 19 / 50 |
|
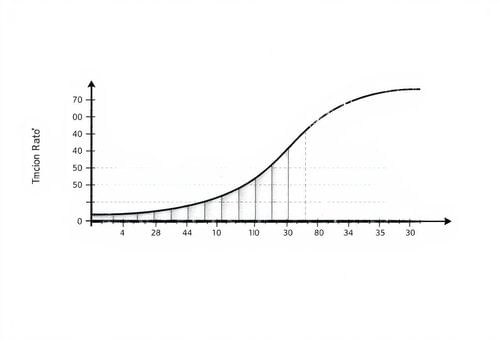
What does the steepness of the slope in a reaction graph indicate about the reaction rate? |
Card: 21 / 50 |
Steeper SlopeReaction Rate and Slope Analysis
|
Card: 22 / 50 |
|
The reaction between calcium carbonate and hydrochloric acid shows the fastest reaction rate at the ___ of the graph. |
Card: 23 / 50 |
|
True or False: A level graph indicates that the reaction is ongoing and products are still being produced. |
Card: 25 / 50 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
FalseChemical Reactions and Graph Interpretation
|
Card: 26 / 50 |
|
Calculate the average rate of reaction if 20 cm³ of carbon dioxide is produced in 20 seconds. |
Card: 27 / 50 |
|
Fill in the blank: The particle theory explains that for a reaction to occur, reactant particles must collide with sufficient ___ to react. |
Card: 29 / 50 |
|
Riddle: I start out plentiful and collide with great frequency, but as I react, I become fewer and slower. What am I? |
Card: 31 / 50 |
|
How does the number of unreacted particles affect the rate of product formation as a reaction progresses? |
Card: 33 / 50 |
DecreasesChemical Reaction Rates
|
Card: 34 / 50 |
|
True or False: Once all reactant particles have reacted, the reaction will continue to produce products indefinitely. |
Card: 35 / 50 |
|
False. Once all reactant particles have reacted, the reaction stops and produces no more products.  |
Card: 36 / 50 |
|
Fill in the blank: In the graph of the copper carbonate and hydrochloric acid reaction, the volume of carbon dioxide is plotted against ___ to analyze the reaction rate. |
Card: 37 / 50 |
|
What happens to the reaction rate as a chemical reaction approaches completion? |
Card: 39 / 50 |
|
The reaction rate decreases, ultimately reaching zero when no reactant particles remain to react.  |
Card: 40 / 50 |
|
True or False: Magnesium powder reacts with oxygen faster than magnesium ribbon because it has a smaller surface area. |
Card: 43 / 50 |
|
In the reaction between hydrochloric acid and sodium thiosulfate, what does the formation of a cloudy precipitate indicate? |
Card: 45 / 50 |
|
Riddle: I can be large or small, but cutting me into pieces makes me react faster. What am I? |
Card: 47 / 50 |
|
Fill in the blank: Smaller pieces of a solid always react faster than larger pieces because cutting a solid increases its total ___ . |
Card: 49 / 50 |