|
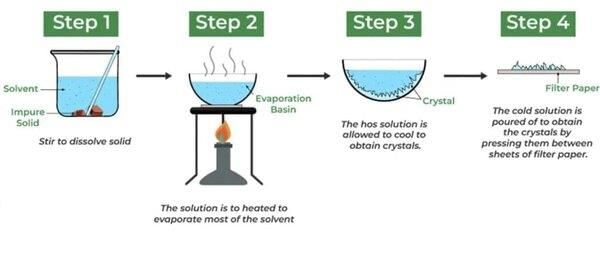
Crystallisation based on the difference in solubility of compounds in a solvent, where a saturated solution forms crystals upon cooling. |
Card: 2 / 30 |
|
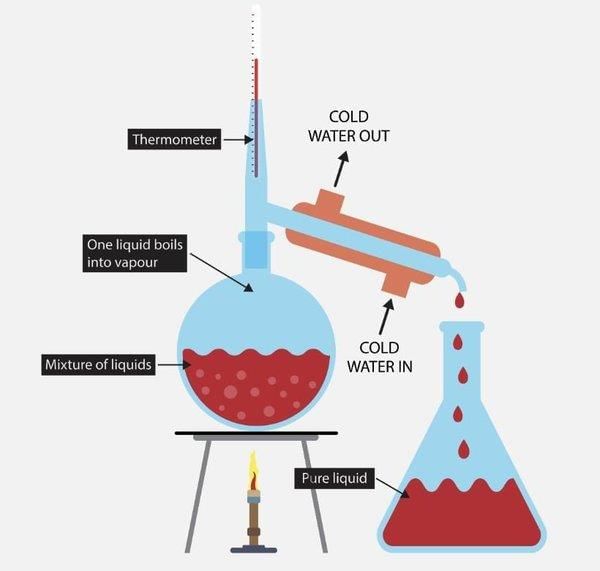
Fill in the blanks: Distillation is used to separate liquids based on their ___ and can be classified into ___ and ___ distillation. |
Card: 3 / 30 |
|
True or False: Sublimation involves a solid turning into a liquid before becoming a vapor. |
Card: 5 / 30 |
|
False. Sublimation involves a solid turning directly into a vapor without passing through the liquid state. 
|
Card: 6 / 30 |
|
Chromatography is primarily used for the separation of complex mixtures into individual components. |
Card: 8 / 30 |
|
Riddle: I can separate liquids based on boiling points, and I can be simple or fractional. What am I? |
Card: 9 / 30 |
|
Sodium metal is used to fuse the organic compound to form soluble sodium salts that can be tested for elements like nitrogen, sulfur, phosphorus, and halogens. |
Card: 12 / 30 |
|
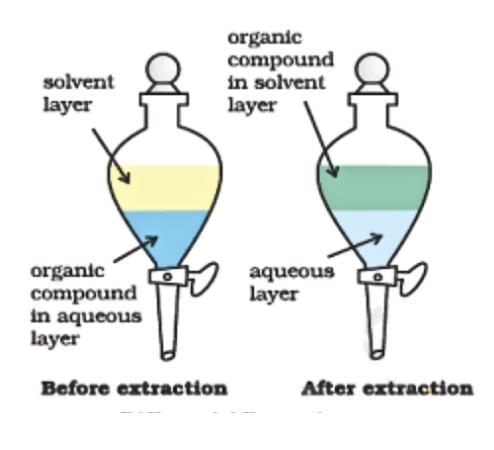
Fill in the blank: In differential extraction, components are separated based on their ___ in different solvents. |
Card: 13 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
A prussian blue color indicates the presence of nitrogen when ferric chloride is added to the filtrate. |
Card: 16 / 30 |
|
True or False: Fractional distillation is effective for separating components with significantly different boiling points. |
Card: 17 / 30 |
|
False. Fractional distillation is used for mixtures with closer boiling points. |
Card: 18 / 30 |
|
What is formed when lead acetate is added to the filtrate in the detection of sulfur? |
Card: 19 / 30 |
|
Fill in the blank: The process that allows a solid to turn into vapor is called ___. |
Card: 21 / 30 |
|
Gas chromatography (GC) uses a gaseous mobile phase to separate volatile compounds. |
Card: 24 / 30 |
|
Riddle: I am a technique that uses differences in solubility and can separate alkaloids. What am I? |
Card: 25 / 30 |
|
In the detection of phosphorus, what color precipitate indicates its presence? |
Card: 27 / 30 |
|
A yellow precipitate indicates the presence of phosphorus when ammonium molybdate is added. |
Card: 28 / 30 |
|
What is the principle behind crystallization, and what are the main steps involved in this purification technique? |
Card: 29 / 30 |
|
Crystallization is based on the difference in solubility of compounds in a solvent. The main steps are: dissolve the impure compound in hot solvent, filter to remove insoluble impurities, cool the solution slowly to allow pure crystal formation, and then filter and wash the crystals with cold solvent to remove soluble impurities. |
Card: 30 / 30 |