|
A chemical bond is the force of attraction between any two atoms in a molecule that maintains stability. |
Card: 2 / 30 |
|
Noble gases have a stable electronic configuration because their outermost shell is ___. |
Card: 3 / 30 |
|
Fill in the blank: The driving force for chemical bonding is the tendency of atoms to attain the stable electronic configuration of the nearest ___. |
Card: 7 / 30 |
|
Riddle: I am a type of bond formed by the complete transfer of electrons from one atom to another. What am I? |
Card: 9 / 30 |
 False. The octet rule applies to all noble gases except helium, which follows the duplet rule. |
Card: 14 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
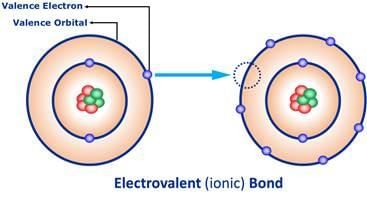
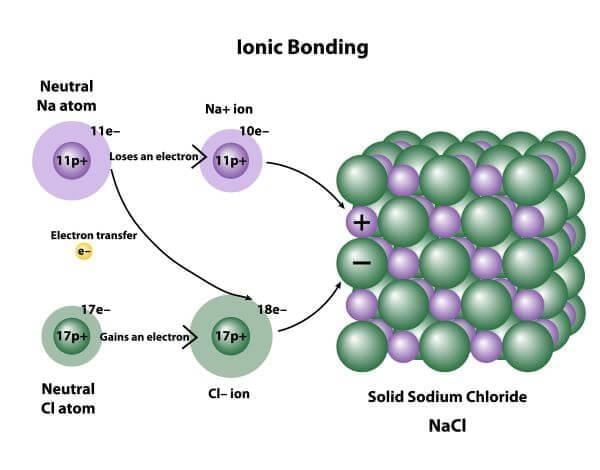
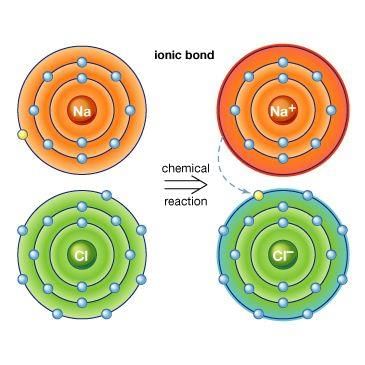
Ionic bonds transfer electrons.
|
Card: 16 / 30 |
|
Fill in the blank: The number of electrons donated or accepted by the valence shell of an atom to achieve stable electronic configuration is called ___ . |
Card: 17 / 30 |
|
Riddle: I am formed when metals lose electrons and non-metals gain them, creating a bond that holds them together. What am I? |
Card: 19 / 30 |
|
Higher values of electron affinity (EA) in non-metallic atoms facilitate the ease of gaining electrons, leading to the formation of anions. |
Card: 22 / 30 |
|
Covalency is the number of electron pairs that an atom shares with one or more atoms to achieve a stable electronic configuration. |
Card: 26 / 30 |
|
What type of covalent compound is characterized by shared electrons that are equally distributed? |
Card: 29 / 30 |