|
The maximum number of electrons that can occupy a shell of number n is given by the formula ___ . |
Card: 1 / 30 |
|
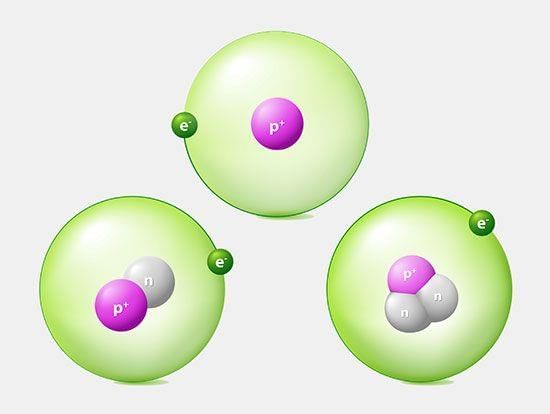
True or False: The mass of an electron is approximately equal to 1/1840 times the mass of a neutron. |
Card: 3 / 30 |
|
False. The mass of an electron is approximately 1/1840 times the mass of a proton, not a neutron.
|
Card: 4 / 30 |
|
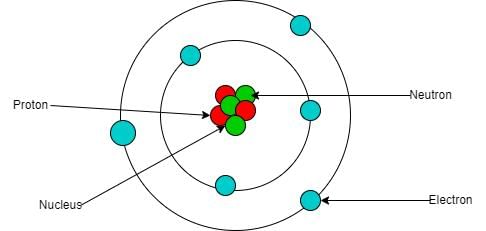
Riddle: I am found in the center of an atom, made of protons and neutrons, I am very small yet hold great power. What am I? |
Card: 7 / 30 |
|
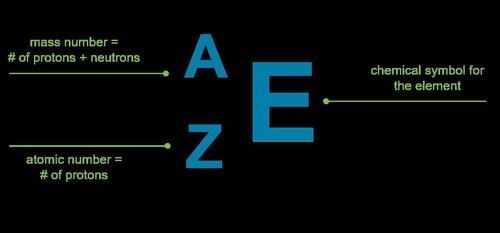
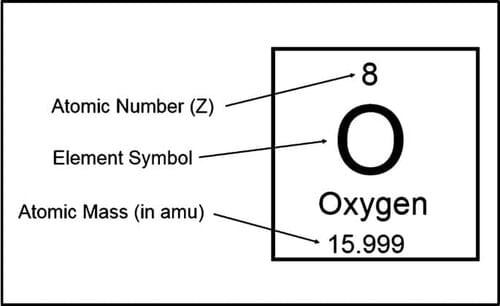
Fill in the blank: The total number of nucleons in the nucleus is called the ___ of the element. |
Card: 11 / 30 |
|
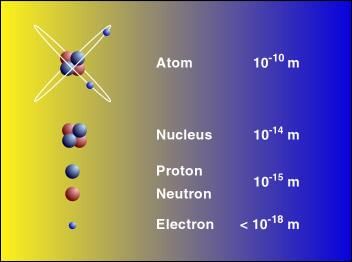
The size of an atom is determined by the radius of the shell of its outermost electron.
|
Card: 14 / 30 |
|
Riddle: I am a particle with no charge, yet I am essential to the nucleus. Without me, the atom would be incomplete. What am I? |
Card: 15 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
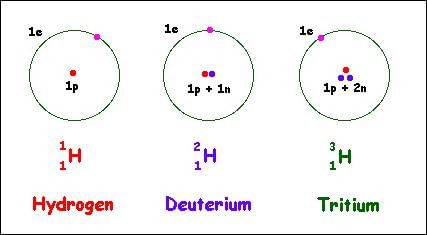
Stable isotopes have equal neutrons and protons.
|
Card: 18 / 30 |
|
Riddle: I am an element's twin, with the same number of protons, but my neutrons differ. What am I? |
Card: 19 / 30 |
|
The number of nucleons in isobars is represented by ___ and their atomic number is represented by ___. |
Card: 25 / 30 |
|
What are the three types of radiation emitted during radioactive decay, and how can they be distinguished? |
Card: 27 / 30 |
|
Three types of radiation are α, β, γ.
|
Card: 28 / 30 |